Antibiotic resistance is one of the most pressing public health challenges of our time. With the alarming rise in bacterial strains that no longer respond to conventional antibiotics, the quest for innovative solutions has never been more critical. Recent research from the University of Illinois Chicago (UIC) offers a glimmer of hope with the development of macrolones, a new class of synthetic antibiotics. These groundbreaking drugs operate by targeting two distinct cellular mechanisms simultaneously, significantly reducing the chance for bacteria to develop resistance.
The implications of this dual-action mechanism are monumental. According to the research, published in *Nature Chemical Biology*, macrolones can obstruct protein synthesis or disrupt DNA integrity. This is not merely a theoretical proposition; the experiments conducted by the UIC team provide compelling evidence that this design effectively complicates the evolutionary pathways of bacteria. By forcing bacteria to develop defenses against two attacks at once, the likelihood of successful mutations that confer resistance dramatically diminishes—by a staggering factor of 100 million, as estimated by the researchers.
The Science Behind Macrolones
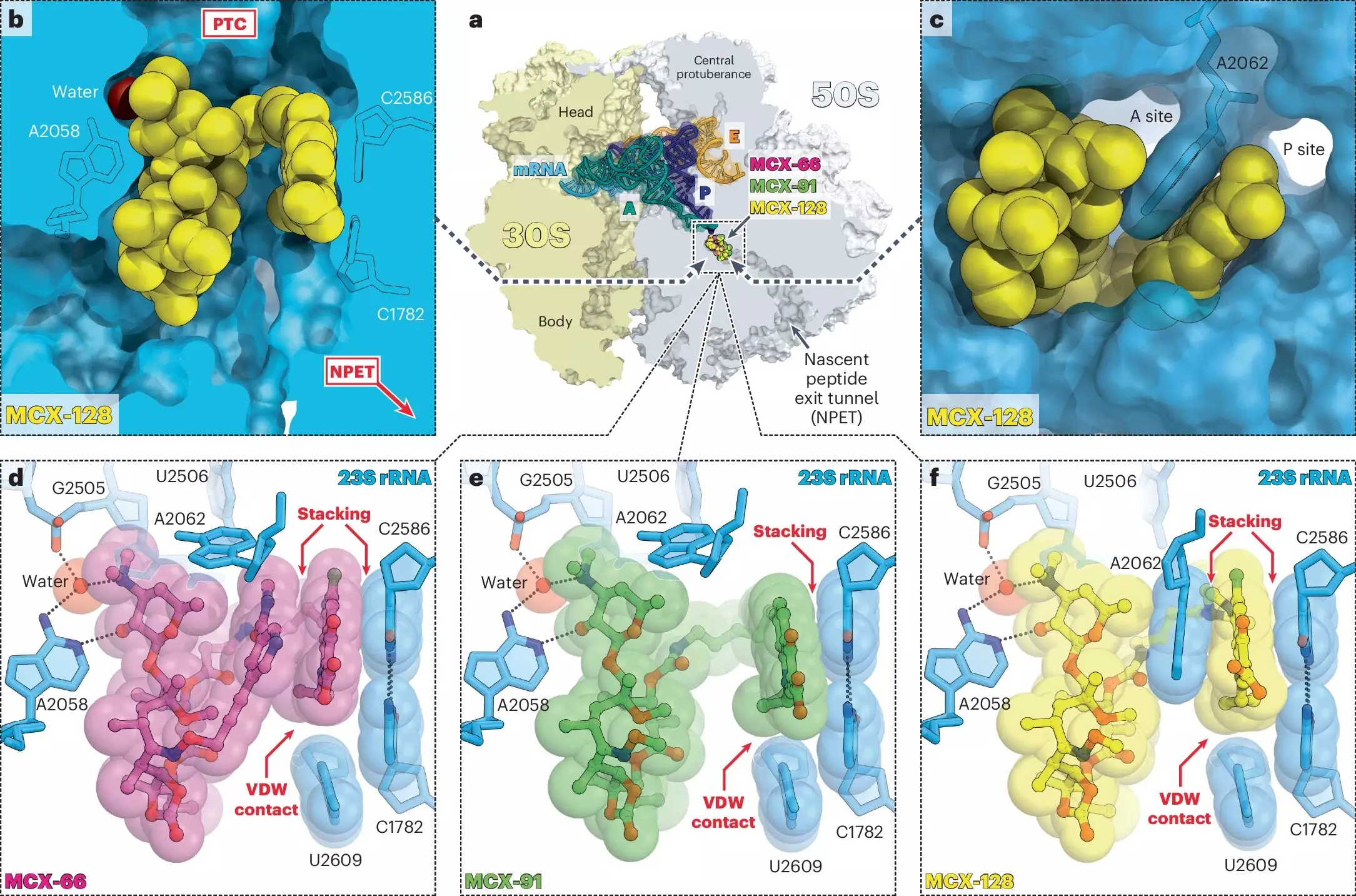
To understand the innovative design of macrolones, one must delve into their structural and functional characteristics. Researchers combined the frameworks of traditional antibiotics—macrolides and fluoroquinolones—that historically target ribosomes and DNA gyrase, respectively. Macrolides, such as erythromycin, inhibit protein synthesis by binding to ribosomes, while fluoroquinolones, like ciprofloxacin, disrupt bacterial DNA replication by targeting specific enzymes.
What sets macrolones apart is their ability to bind both targets more effectively than their traditional counterparts. Professor Yury Polikanov’s laboratory at UIC played a crucial role in investigating how these synthetic agents interact with ribosomes. They found that macrolones not only bind tightly to ribosomal structures but are also effective against strains of bacteria that have developed resistance to macrolides. This is a significant achievement because it shows that these antibiotics can operate in contexts where older antibiotics have failed, offering hope for treating stubborn infections.
The Interdisciplinary Approach to Drug Development
The development of macrolones underscores the importance of collaborative research in tackling complex medical challenges. The project showcases a blend of expertise from different scientific disciplines, emphasizing how respective fields—pharmaceutical sciences, structural biology, and molecular biology—can intersect to yield innovative solutions. Researchers, including Alexander Mankin and Nora Vázquez-Laslop, noted that their collaborative environment at the UIC Molecular Biology Research Building fosters an ecosystem where ideas can flourish, accelerating the journey from concept to application.
The interdisciplinary nature of this study also informs a broader narrative about the future of antibiotic research. It highlights the necessity for chemists and biologists to closely collaborate in order to refine these macrolone compounds to strike a balance in targeting both cellular processes effectively. The findings suggest that the continuous optimization and exploration of these drugs could lead to new standards in how we approach antibiotic development.
Challenges Ahead and the Road to Implementation
While macrolones present a groundbreaking approach, the road to practical application is fraught with obstacles. Beyond the excitement surrounding their dual mechanisms, researchers will need to undertake extensive studies to confirm their efficacy in clinical settings and ensure their safety for human use. Furthermore, the challenge of regulatory approval remains a formidable hurdle that will necessitate rigorous testing and validation processes.
Moreover, the emergence of new varieties of multi-drug resistant bacteria could raise questions about the longevity of this approach. Though the science behind macrolones is robust, it opens up discussions around the sustainability of antibiotic development in an era where bacterial adaptation is swift and unpredictable. The quest to stay ahead in this ever-evolving arms race between bacteria and antibiotics demands continuous innovation and responsiveness in research methodologies.
Research like this demonstrates that while we face significant challenges from resistant pathogens, we are also on the cusp of new breakthroughs. With a commitment to interdisciplinary collaboration and sustained investment in antibiotic research, innovations such as macrolones could pave the way for a new era in the fight against infectious diseases.