In the rapidly advancing field of biotechnology, the ability to accurately observe and quantify biomolecules within living cells is paramount for the evolution of therapeutic strategies, particularly in drug development and cell therapies. Traditional imaging techniques often fall short due to inherent limitations in their resolution and the impact of the cell’s aqueous environment. Recently, groundbreaking research from the National Institute of Standards and Technology (NIST) has introduced an innovative method that leverages infrared (IR) light to penetrate these barriers and deliver clearer insights into biomolecular composition.
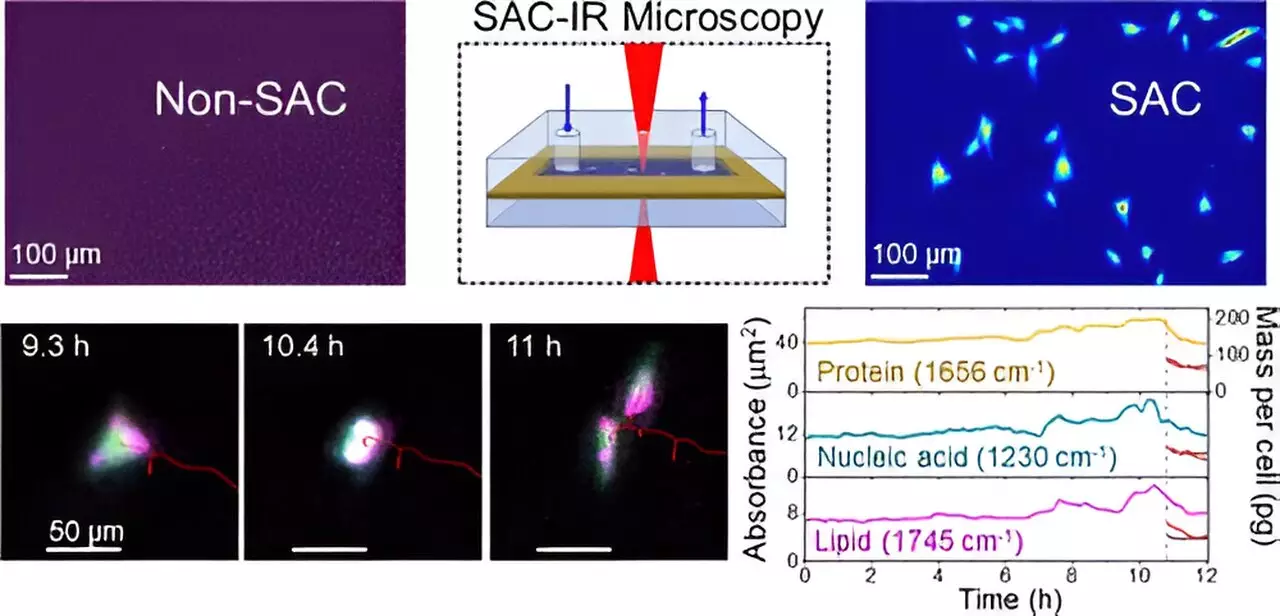
Previously, the strong absorption of infrared radiation by water—a critical component of biological cells—posed a significant hurdle for researchers attempting to visualize biomolecules. Understanding this challenge is akin to trying to catch a glimpse of an airplane obscured by bright sunlight. Without the right tools, the airplane remains hidden. NIST chemist Young Jong Lee and his team have developed a unique approach called Solvent Absorption Compensation (SAC), which effectively “uncloaks” the water absorption effects, revealing the relevant protein signals.
The SAC technique integrates an optical element to filter out the interference caused by water, allowing for a clearer analysis of biomolecules like proteins, lipids, and nucleic acids. This pioneering method culminates in what is termed SAC-IR microscopy. This label-free, non-invasive technique frees researchers from the constraints and potential biases introduced by dyes and fluorescent markers, fostering more consistent and reliable results across different labs.
Implications for Biomanufacturing and Drug Development
The implications of the SAC-IR technique are far-reaching. One of its primary advantages is the ability to track changes within living cells in real-time, which could dramatically accelerate the pace of biomanufacturing and drug development. For instance, during a comprehensive 12-hour study utilizing fibroblast cells, NIST researchers successfully identified groups of biomolecules at various phases of the cell cycle, including during crucial stages like cell division.
The study breaks new ground in quantitative measurements of biomolecules, allowing for an analysis of the absolute mass of proteins and other cellular components. As this technique gains momentum, it has the potential to establish standardized protocols necessary for biomolecular assessments, which could benefit both academic research and clinical applications.
Perhaps one of the most compelling applications of the SAC-IR methodology lies within the realm of cancer therapy. By enabling researchers to evaluate modifications in the patient’s immune system cells—after they have been engineered to better target cancer cells—this new imaging technology offers crucial insights. Questions pertaining to the safety and efficacy of these altered cells can now be addressed through precise measurements that were previously unattainable.
Moreover, for drug discovery processes, SAC-IR opens doors for high-throughput screening of candidate drugs. By analyzing the interactions and concentration levels of various biomolecules across a multitude of individual cells, researchers can assess how different cellular environments respond to new drugs, potentially expediting the journey from laboratory discovery to clinical application.
While the initial findings from NIST are promising, there are ongoing aspirations to enhance the SAC-IR method further. Researchers aim to extend its capabilities to measure additional key biomolecules, such as DNA and RNA, thus broadening the scope of cellular analysis. These capabilities could lead to more profound understandings of fundamental cell biology principles, including determining specific biomolecular signatures associated with cell health or viability.
Understanding cell viability has numerous practical implications, especially with regard to cell preservation techniques. As Lee mentioned, questions surrounding the optimal methods for freezing and thawing preserved cells could be addressed through the new measurement capabilities offered by SAC-IR. This could result in advances that enhance the viability of cells post-thaw, contributing to better patient outcomes in regenerative medicine.
The development of the SAC-IR method represents a significant leap forward in the field of biomolecular imaging, facilitating new avenues for research that were previously hindered by the challenges posed by cellular water. As this technology continues to evolve and gain traction within various scientific disciplines, it promises to serve as a foundational pillar for future innovations in health and medicine, drug development, and our understanding of cellular dynamics. By bridging the gaps in biomolecular quantification, this advanced imaging technique stands to transform the landscape of biotechnology for years to come.