The urgency for sustainable practices in chemical engineering has never been more pronounced, as global industry increasingly seeks to reduce waste and minimize reliance on non-renewable resources. Recently, researchers from the University of Illinois Urbana-Champaign have made a groundbreaking advancement in this area by developing a novel polymer that selectively attracts specific substances from solutions when electrically activated. This innovation, highlighted in the journal JACS Au, presents a significant leap towards sustainable chemical separation through the mechanism of halogen bonding.
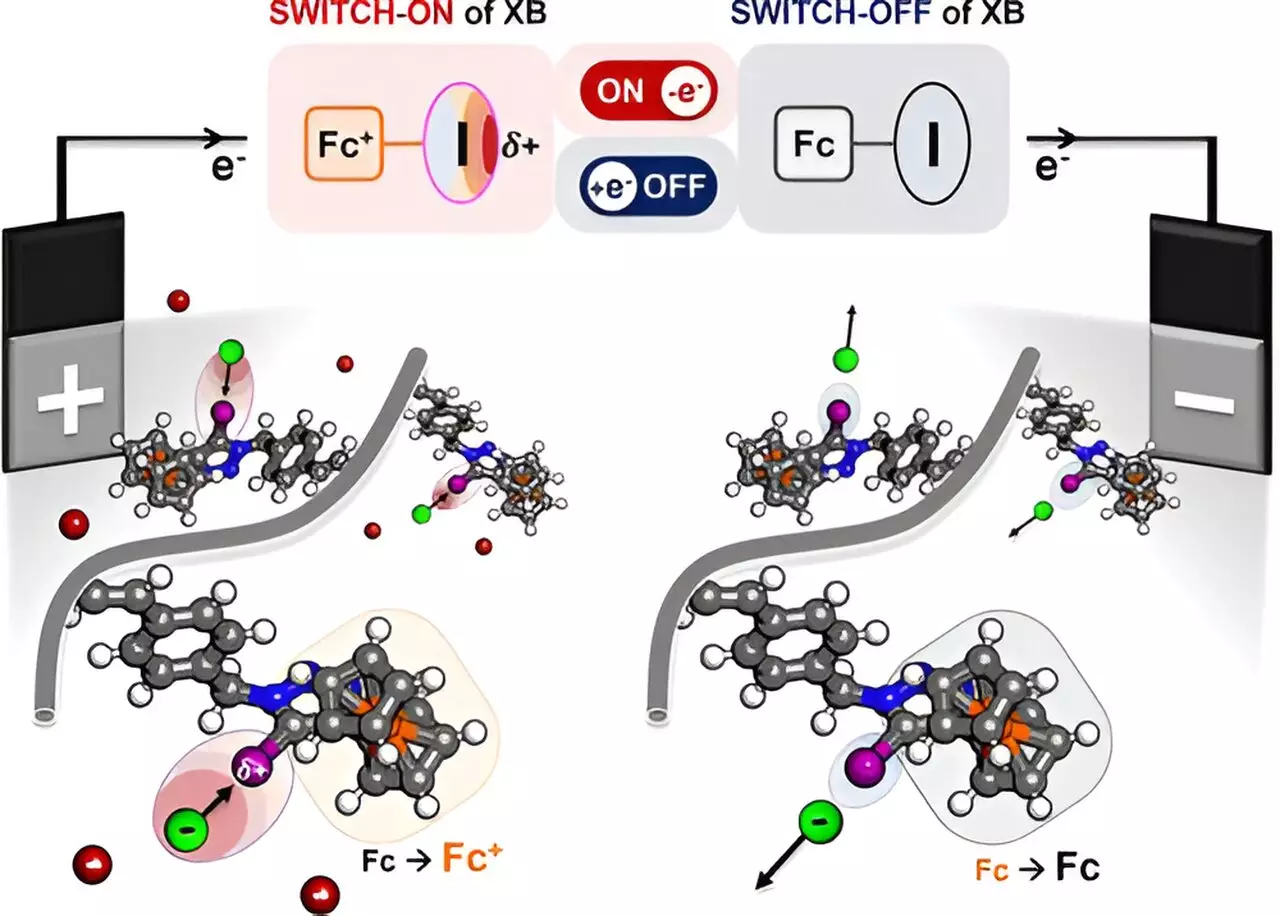
The polymer engineered by the researchers utilizes a unique principle: modulating the charge density of a halogen atom in response to electrical stimuli. This process allows the polymer to attract targeted ions and organic molecules in a highly selective manner. Traditional methods of chemical separation, such as thermal processes and membrane filtration, are often inefficient and generate substantial waste. With the new electrochemical approach, waste generation could be significantly reduced as the method uses an electric field to facilitate the separation of only desired components from a mixture, creating a more sustainable alternative.
The principle behind this selective attraction lies in a phenomenon known as halogen bonding. When electrical activity is applied, the polymer’s halogen atom develops a positive charge, creating a “sigma hole.” This charged area enables the polymer to selectively bind to negatively charged ions present in the solution, extracting them with precision.
The implications of this research are vast, particularly for sectors like pharmaceuticals and chemical synthesis, where effective separation of compounds is essential. Traditional separation methods often lead to resource intensiveness and environmental liabilities due to the production of chemical byproducts. In contrast, the ‘electric sponge’ concept can revolutionize the way industries approach chemical synthesis and separation. For instance, by employing an electrochemical mechanism that selectively extracts target components without affecting other substances, companies can enhance the purity of their products while minimizing waste.
Xiao Su, the project lead and professor of chemical and biomolecular engineering, likened the polymer to a sponge specifically designed to absorb only the desired chemical from a mixture. This innovative perspective underlines the potential for the polymer to transform established practices across multiple sectors.
In a series of experiments, the research team tested the effectiveness of the polymer in various organic solutions. They successfully confirmed the presence of halogen bonding through advanced analytical techniques, including nuclear magnetic resonance (NMR) and Raman scattering experiments. Nayeong Kim, a graduate student involved in the project, noted that their work not only validated the concept of selective separation but also opened the door for further research in this underexplored area of chemistry.
Collaboration with computational experts led by Professor Alex Mironenko played an essential role in understanding the operational mechanics of the redox center within the polymer. This comprehensive approach allowed the researchers to fine-tune the polymer’s properties and confirm the effectiveness of halogen bonding in attraction to specific ions.
As the initial proof-of-concept has been established, the next steps will involve refining the technology for practical applications. Researchers aim to explore scalable manufacturing processes and investigate systems for continuous electrosorption. The successful scaling of this technology will be pivotal in realizing its implementation in industrial settings, further enhancing the practicality of the polymer for widespread use in reducing waste generated by traditional separation methods.
Overall, the innovative approach to electrochemical separation developed by the University of Illinois team signifies a promising future for chemical engineering practices. It serves as an exciting example of how fundamental research can lead to applied technologies that not only address industrial issues but also contribute significantly to environmental sustainability. As research progresses, the potential for this technology to improve the efficiency and sustainability of chemical manufacturing processes is substantial, paving the way for greener industry standards.