Fuel cells represent a groundbreaking technology in the quest for clean energy, generating electricity through electrochemical reactions rather than combustion. This makes them a vital alternative to conventional fossil fuel sources, which significantly contribute to environmental pollution. With applications in electric vehicles, portable devices, and industrial equipment, fuel cells are pivotal in transitioning to sustainable energy solutions. However, the technology’s growth has been impeded by reliance on costly materials, specifically precious metal catalysts. This limitation has sparked a search for alternative solutions, particularly in the development of anion-exchange-membrane fuel cells (AEMFCs).
While AEMFCs offer a promising pathway to harness cleaner energy, their widespread adoption has been hampered by the reliance on expensive catalysts. The majority of existing systems utilize precious metals that create economic barriers for large-scale applications. However, researchers are actively pursuing innovative strategies to overcome these challenges by engineering fuel cells that employ more abundant and less costly materials. Among these efforts, the focus on non-precious metals has become particularly significant. Yet, the transition to these materials has been fraught with difficulties. Studies have shown that many of these catalysts suffer from self-oxidation, which leads to irreversible degradation of the fuel cell performance.
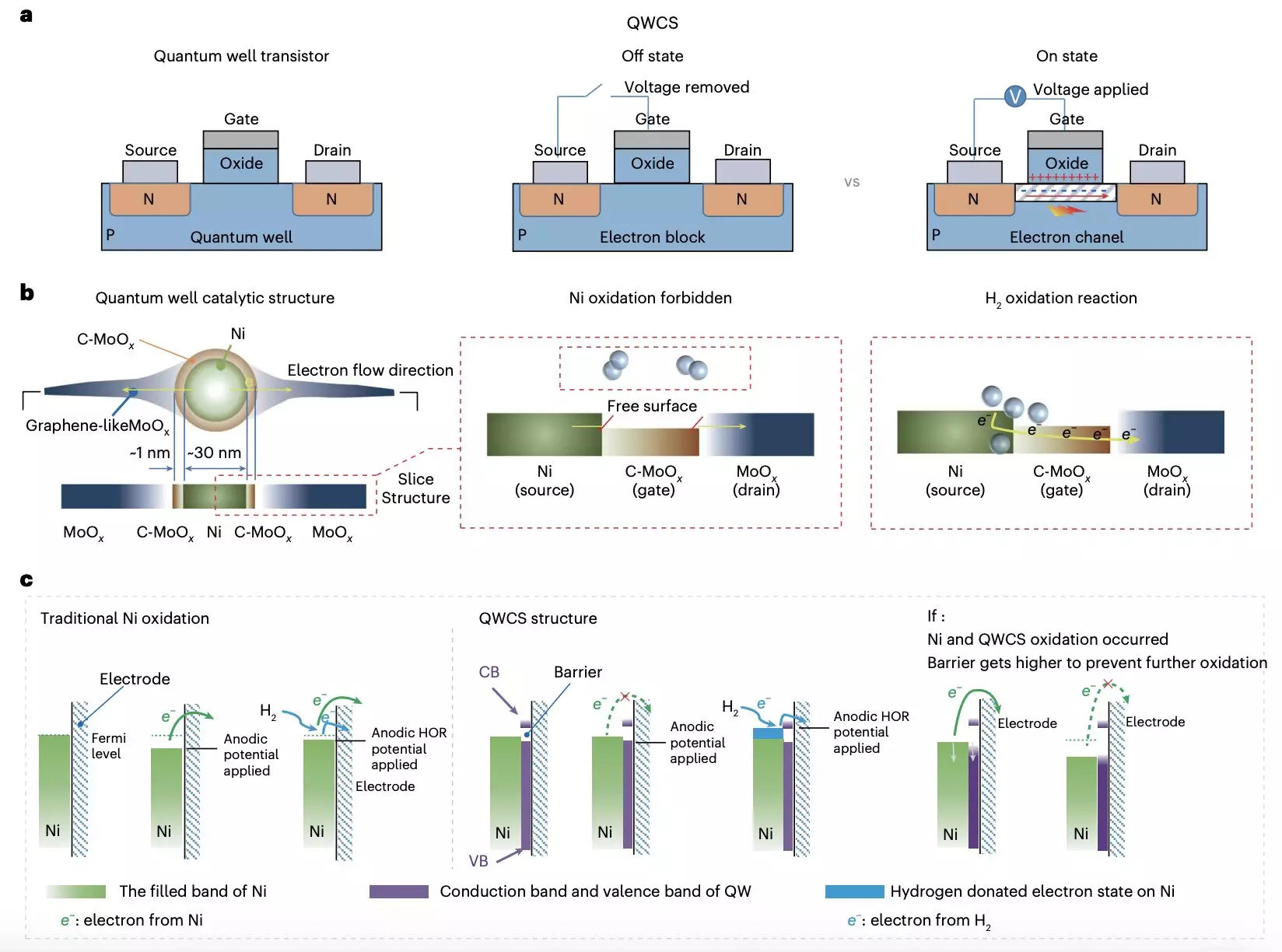
Recent research from Chongqing University and Loughborough University has unearthed a potential solution to this critical issue. By introducing a novel quantum well-like catalytic structure (QWCS), the team seeks to address the inherent vulnerabilities of metallic nickel when utilized as a catalyst for hydrogen oxidation reactions. Published in “Nature Energy,” this innovative structure offers a promising approach to ensure the durability and effectiveness of AEMFCs. The QWCS includes quantum-confined metallic nickel nanoparticles, which have been designed to maintain their metallic state while effectively catalyzing the required reactions.
The QWCS model pioneered by the research team integrates atomically confined nickel nanoparticles within a specialized heterojunction, composed of carbon-doped MoOx (C-MoOx) and amorphous MoOx. This configuration has been ingeniously engineered to create low and high energy barriers, enhancing the efficiency of electron transfer in the catalytic process. The Ni@C-MoOx catalyst serves as a beacon of resilience, allowing for the selective transfer of electrons generated from the hydrogen oxidation reaction while simultaneously preventing the unwanted transfer of electrons from the nickel catalyst into the QWCS.
The implications of this research are profound. The Ni@C-MoOx catalyst exhibited impressive performance in sustained tests, showcasing remarkable catalytic stability even after enduring 100 hours of continuous operation under challenging conditions. An alkaline fuel cell constructed using this catalyst achieved a specific power density reaching 486 mW mgNI-1, demonstrating exceptional reliability during repeated operational cycles. The significant resilience of this fuel cell against performance decline illustrates the potential impact of the QWCS-based catalyst design on practical applications.
What sets the QWCS apart is its unique ability to manage electron barriers effectively. By providing a barrier of 1.11 eV for the electrons of Ni nanoparticles, the structure enhances stability while allowing electrons released from hydrogen oxidation to traverse the barrier effortlessly. This ability to regulate electron flow is critical in preventing self-oxidation processes, thereby extending the functional lifespan of the fuel cells.
The introduction of the QWCS presents exciting possibilities for the future of clean energy technologies. By utilizing a cost-effective non-precious metal catalyst that exhibits resilience against degradation, researchers are paving the way for more accessible fuel cell solutions. Beyond immediate applications in AEMFCs, the design principles established through this research could spur the development of other catalytic systems aimed at leveraging quantum confinement.
This innovative approach not only highlights the potential for sustainable energy solutions but also reinforces the notion that with creativity and rigorous scientific inquiry, it is possible to overcome significant technological challenges. The path towards enhanced fuel cells that are both affordable and efficient is becoming clearer, and the research undertaken by these teams marks a crucial step in making sustainable energy more achievable in our daily lives.