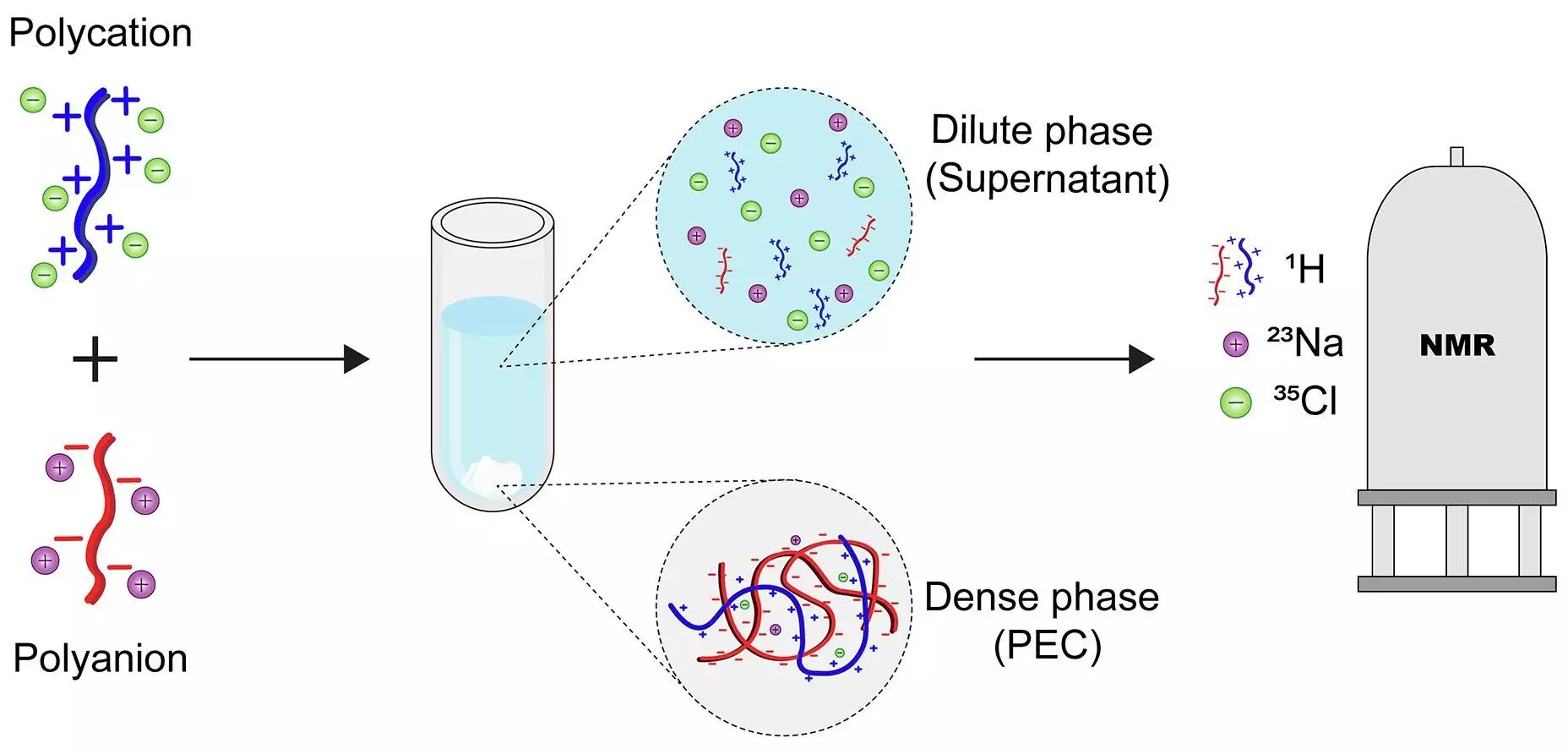
For decades, the scientific community has been captivated by the enigmatic nature of polyelectrolyte complexes—interactions between oppositely charged polymers that spontaneously form ordered structures. These complexes hold the potential to revolutionize a spectrum of industries, from water purification to targeted drug delivery, owing to their unique ability to assemble and disassemble in precise manners. However, the true challenge has been deciphering the internal distribution of these charged entities post-formation—a puzzle that has hindered efforts to optimize their functionalities.
The recent advent by researchers at the University of Twente marks a pivotal turn in this quest. By leveraging nuclear magnetic resonance (NMR) spectroscopy, they have developed a robust, rapid, and non-invasive method to map exactly where each component—be it the polymer chains or their counterions—settles within the phase-separated system. This technological leap not only fills a long-standing knowledge gap but also opens doors to a new era of rational design for polyelectrolyte-based materials.
From Concept to Precision: The Power of Nuclear Magnetic Resonance
At the heart of this innovation lies the application of NMR spectroscopy, a technique traditionally associated with organic and medicinal chemistry, now ingeniously adapted for complex polymer systems. The researchers’ protocol circumvents previous limitations by allowing for a comprehensive, swift, and label-free analysis. Reading the subtle magnetic signals emitted by atomic nuclei, this method precisely quantifies the distribution of each component across different phases, revealing the inner architecture of these complex systems.
What makes this development transformative is its speed and simplicity. Completing a full analysis in less than 40 minutes signifies a game-changing advantage for research and industry alike. Previously, such detailed insights required cumbersome, time-consuming procedures that often disrupted the delicate equilibrium of the complexes themselves. Now, scientists can obtain real-time, high-resolution snapshots of the internal distribution, empowering them to fine-tune formulations with unprecedented accuracy.
Implications for Material Innovation and Natural Processes
This breakthrough transcends mere academic curiosity; it embodies a critical step toward engineering smarter, more efficient materials. Porous membranes, ion exchange systems, and extraction media—cornerstones of environmental and industrial technology—stand to benefit immensely. With precise knowledge of component distribution, researchers can manipulate conditions to maximize performance, durability, and selectivity in these materials.
Furthermore, the implications extend into biological realms. Since polyelectrolyte complexes are abundant in nature—forming the foundation of cellular structures, DNA packaging, and mucosal barriers—this method offers a window into natural processes that have evolved over millions of years. By understanding how charge and component distribution guide formation and function naturally, scientists can mimic or even enhance these biological systems in biomedical applications.
However, as with any groundbreaking technology, critical scrutiny reveals potential challenges. The specificity of NMR might be limited when dealing with highly complex or heterogeneous mixtures. Additionally, translating these laboratory successes into industrial-scale applications may require further refinement to handle larger sample volumes or more diverse systems.
In essence, the work from Twente University exemplifies a bold stride toward mastering the microscopic world of charged polymers. It underscores the importance of innovative analytical techniques in transforming theoretical concepts into practical solutions. As we edge closer to designing materials with atomic precision, it becomes both evident and exciting that the future of material science hinges on our ability to see the unseen—and to understand the unseen, we need tools like this advanced NMR methodology.