Ruddlesden-Popper compounds are garnering serious attention in the academic and industrial communities alike. Their unique layered structure opens avenues for impactful applications, significantly in fields such as superconductivity, catalysis, and photovoltaics. Traditionally, these materials have primarily included various oxides and halides, yet the synthesis of nitrides, a class that promises exceptional properties, remained elusive. The recent groundbreaking work led by Dr. Simon Kloß and his team from LMU challenges this stagnation and introduces a new era for Ruddlesden-Popper materials.
The Challenge of Synthesis
The synthetic difficulty associated with Ruddlesden-Popper nitrides cannot be understated. Nitrogen’s inherent stability as a diatomic molecule (N2) and its low electron affinity create obstacles that have deterred scientists for years. In essence, the properties that make nitrogen so favorable in a variety of contexts—such as its abundance and chemical stability—also contribute to the complexity involved in developing nitrogen-rich compounds. Hence, while the scientific community had high expectations regarding the properties of Ruddlesden-Popper nitrides, their realization remained purely theoretical until now.
A Breakthrough Methodology
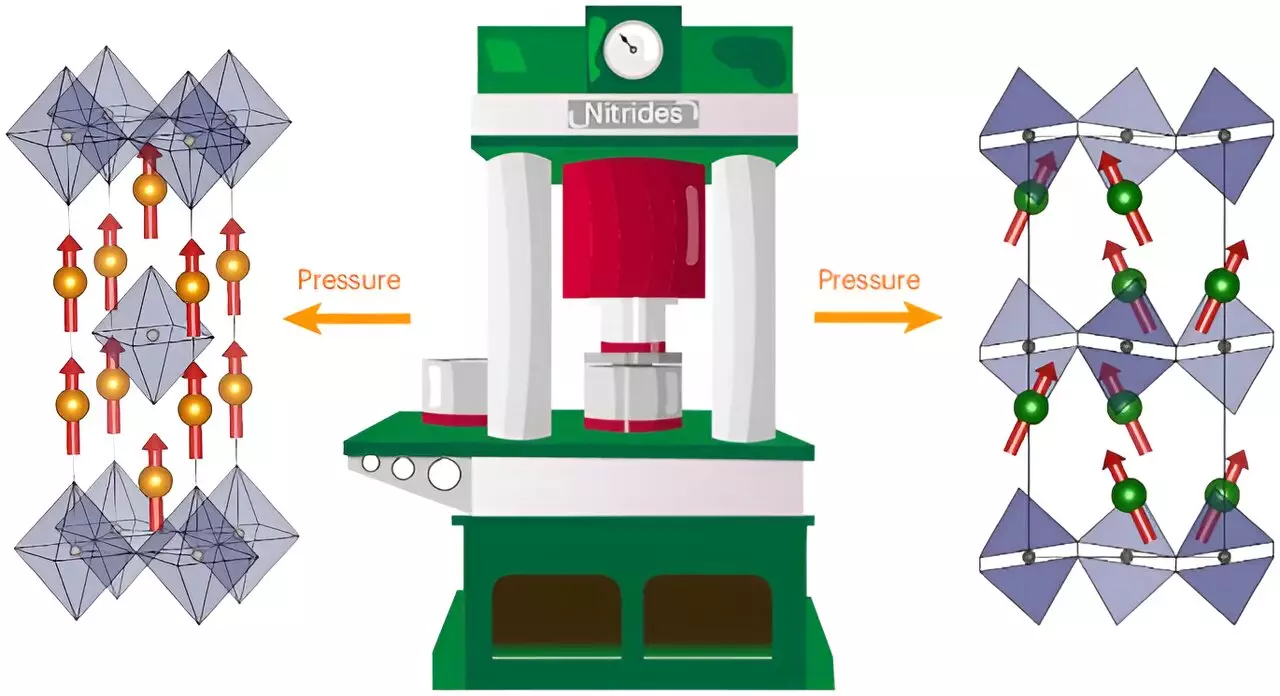
The ingenuity of Kloß’s synthetic pathway cannot be overlooked. By employing extreme conditions and utilizing large-volume presses to apply pressures reaching 8 gigapascals (or around 80,000 bars), the researchers successfully navigated the unique challenges presented by nitrogen chemistry. This breakthrough in synthesis is not merely a technical achievement; it opens the door to exploring the landscape of Ruddlesden-Popper nitride compounds systematically. The choice to use sodium azide as an active nitrogen source speaks to both the creativity and resourcefulness of the research team, highlighting their understanding of complex inter-element relationships.
Exciting Findings from Initial Syntheses
The first results from this ambitious endeavor yield three exciting new compounds, including cerium-tantalum nitride (Ce2TaN4) and praseodymium and neodymium rhenium nitrides (Ln2ReN4). The rich structural, electronic, and magnetic properties exhibited by these compounds underscore their potential significance in various applications. For instance, the neodymium compound stands out as a hard ferromagnet, an attribute that could revolutionize the way we understand magnetic materials and their applications in technology. Furthermore, the tantalum compound’s semiconductor properties position it as a compelling candidate for energy conversion applications—ranging from solar technologies to electronic devices.
Future Directions in Material Science
Kloß’s assertion that this synthetic method may lead to discovering various Ruddlesden-Popper nitride compounds and their derivatives sparks optimism among materials scientists. The potential for this novel approach to facilitate the development of new materials can transform our understanding of superconductivity and energy-efficient technologies. Additionally, the promise shown in magnetic characteristics of the newly synthesized compounds could lead to innovations in data storage, computing, and other fields reliant on magnetism.
The strides made in the synthesis of Ruddlesden-Popper nitrides challenge previous limitations and illuminate an exciting future in materials science. As we stand on the brink of exploration, the implications for technology across multiple sectors are significant, inviting further research that could redefine how we harness materials for modern-day applications.