Organic chemistry has always been a complex and nuanced field, often entangled in the challenges of molecular synthesis. However, recent research led by Professor Max Martin Hansmann from the Department of Chemistry and Chemical Biology has introduced a groundbreaking reagent that may alter the landscape of how carbon atoms can be added to molecules. Published in the prestigious journal Science, this advancement is more than just a scientific milestone; it promises to reshape the methods researchers employ to create complex pharmaceuticals with greater efficiency and precision.
The synthesis of organic molecules is frequently marred by tedious protocols and lengthy reaction sequences. Professor Hansmann and his team are tackling these limitations head-on by developing a reagent that allows for the selective introduction of carbon atoms—an endeavor previously thought to be cumbersome and fraught with complications. The research is both promising and an indication of the innovative potential that modern chemistry holds for the pharmaceutical industry. The ability to access complex compounds in a shorter synthetic pathway could accelerate the development of therapies and treatments in ways we can only begin to imagine.
Innovative Methodology: The Elegance of Carbones
A vital aspect of this research lies in the innovative approach to stabilizing carbon atoms through coordination with two electron-donating groups. This sophisticated technique has given rise to a unique species known as carbones, which had largely remained underexplored until now. By effectively flanking the carbon atom with labile groups, Professor Hansmann’s team has navigated a long-standing barrier in the organic chemistry community.
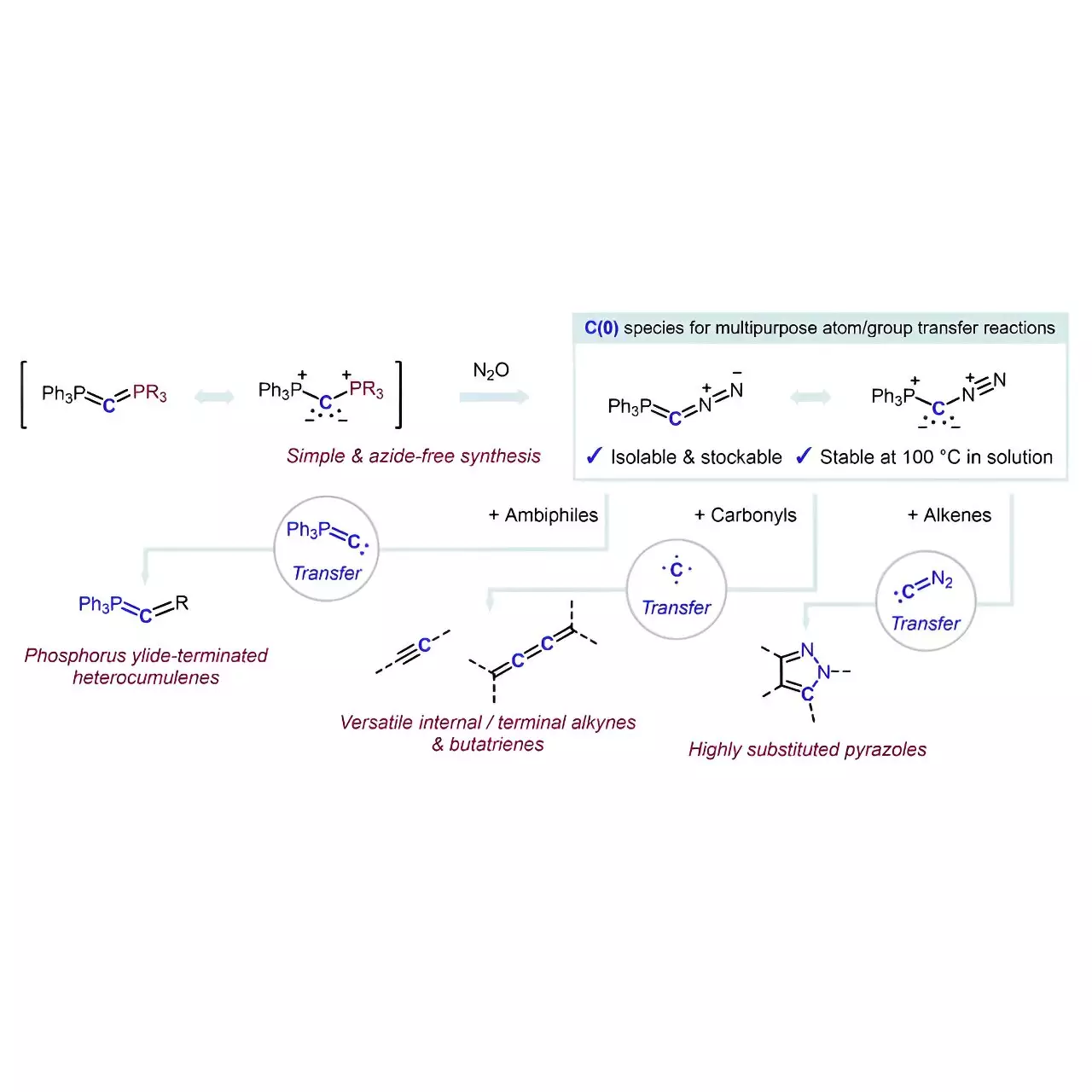
Additionally, the method by which the reagent Ph3P=C=N2 was synthesized is noteworthy for its simplicity and efficiency. By employing a PPh3/N2 exchange reaction with carbodiphosphorane and nitrous oxide, the researchers have circumvented the traditional safety risks associated with azides, which often form part of diazo compounds utilized in similar reactions. This not only highlights the ingenuity of the researchers but also sets a new standard for safety and procedural elegance in the lab.
High Selectivity: The Future of Carbon Atom Transfer
One of the truly remarkable aspects of this new reagent is its high selectivity in transferring carbon fragments. It serves as a multifunctional agent capable of executing diverse chemical reactions without necessitating additional additives. This translates into substantial time savings and reduced complexity, allowing chemists to focus on exploring and harnessing the potential of their desired compounds rather than wrestling with cumbersome synthetic processes.
In their findings, the team elucidated how the reagent can be employed in various reactions, from generating phosphorus ylide-terminated heterocumulenes to transferring carbon atoms to alkenes that result in multi-substituted pyrazoles. Furthermore, reactions involving carbonyl compounds yield diverse alkynes or butatrienes—two classes of compounds with significant implications for drug development and materials science.
Implications for Research and Industry
The implications of this research extend far beyond the confines of academic circles. With the pharmaceutical industry continually striving for quicker and more reliable methods for compound synthesis, this advancement is poised to make a substantial impact. For chemists, the ability to incorporate carbon atoms selectively opens doors to new molecular architectures that could lead to novel therapeutic agents.
Moreover, the exploration of carbones as a carbon atom source could drive further innovation within organic chemistry, encouraging researchers to take on challenges previously deemed too risky or complicated. Professor Hansmann’s optimism regarding new applications for these reagents suggests a fruitful horizon for future research in complex molecular structures.
The pioneering work led by Professor Max Hansmann and his team is set to redefine the methodologies of organic chemistry, promoting a future where efficiency, safety, and precision in the synthesis of complex molecules are not merely aspirational but achievable realities. With such transformative potential at our fingertips, the scientific community must recognize and nurture these advancements for the benefit of both research and society at large.