Noble gases have long been celebrated for their remarkable stability and lack of chemical reactivity. Characterized by a complete valence shell, these elements—including helium, neon, argon, krypton, xenon, and radon—have historically been considered inert. This perspective shifted over 60 years ago when Neil Bartlett, a pioneering chemist, successfully synthesized the first noble gas compound, xenon hexafluoroplatinate (XePtF6), an orange-yellow solid that marked a monumental milestone in chemical research. Bartlett’s achievement, recognized as an International Historic Chemical Landmark, opened a new realm of possibility regarding the reactivity and usefulness of noble gases, paving the way for future exploration.
Despite notable advances since Bartlett’s initial discovery, the study of noble gas compounds remains fraught with challenges. The inherent reactivity of these compounds, particularly in the presence of moisture, poses significant obstacles for chemists. Traditional techniques like single-crystal X-ray diffraction require the growth of sizable, high-quality crystals, yet noble gas-containing compounds often prove difficult to crystallize satisfactorily. As a result, many of their structures, and by extension, their functions, remain poorly understood.
The current state of research has revealed various noble gas compounds synthesized in labs worldwide, yet detailed characterizations remain elusive. Moisture sensitivity calls for the utilization of sophisticated techniques and specialized equipment, which restricts the ability to comprehensively analyze these structures.
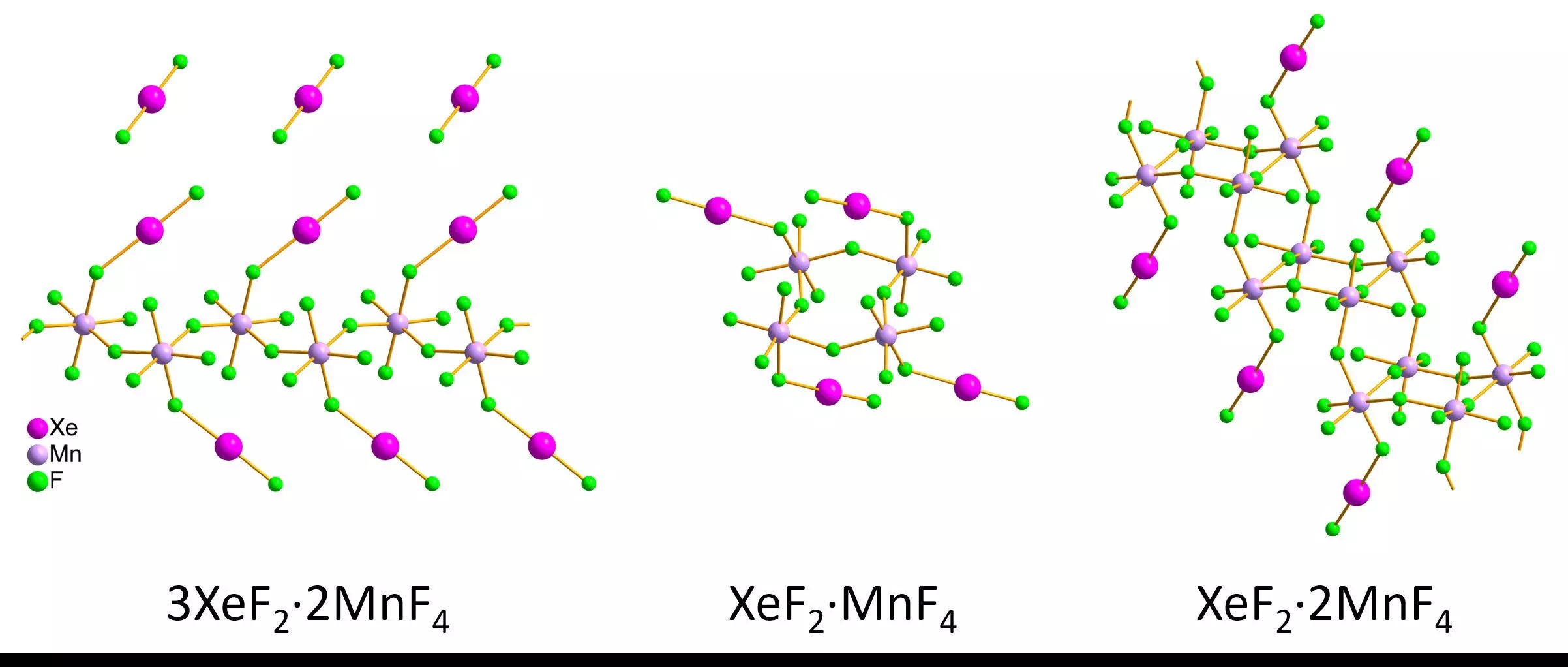
In response to these challenges, researchers have turned to innovative methodologies, notably 3D electron diffraction, which has the potential to unlock new insights into noble gas compounds. A recent study led by Lukáš Palatinus and Matic Lozinšek endeavored to explore this technique’s applicability to xenon-containing crystallites. By synthesizing and analyzing several xenon difluoride-manganese tetrafluoride compounds, the team demonstrated the feasibility of using 3D electron diffraction to examine tiny crystallites, circumventing the limitations posed by larger crystal requirements.
In their experiments, the researchers utilized a carefully controlled environment to stabilize the samples, employing liquid nitrogen to cool the holder and multiple protective layers during transfer to a transmission electron microscope. This systematic approach enabled them to measure the bond lengths and angles of the xenon-fluoride and manganese-fluoride interactions accurately.
The results from Palatinus and Lozinšek’s research were promising. They found that the structures of the small nanoscale crystals obtained via 3D electron diffraction were comparable to those acquired through traditional methods, despite minor discrepancies. Their findings revealed intriguing structural configurations for the xenon compounds, including infinite zigzag chains, defined rings, and staircase-like double chains.
This breakthrough suggests that 3D electron diffraction could be a game-changer for the field of noble gas chemistry, offering a viable path to elucidate the structures of long-elusive compounds such as XePtF6 and other air-sensitive materials. The technique stands to widen our understanding of noble gases within both academic and applied contexts, potentially leading to innovative applications in fields ranging from materials science to environmental monitoring. The ability to characterize these compounds more reliably promises a new era of exploration, turning the seemingly inert into the remarkably reactive.