In a significant advancement for environmental science, a research team has unveiled a novel method for tackling water pollution through the innovative use of single-atom catalysts (SACs). This research, conducted by scientists from the University of Science and Technology of China and the Suzhou Institute for Advanced Study, was recently published in the esteemed journal *Nature Communications*. The team focused on enhancing the efficacy of Fenton-like processes, which are traditionally employed to degrade harmful substances in water, showcasing the potential of SACs to transform water purification practices.
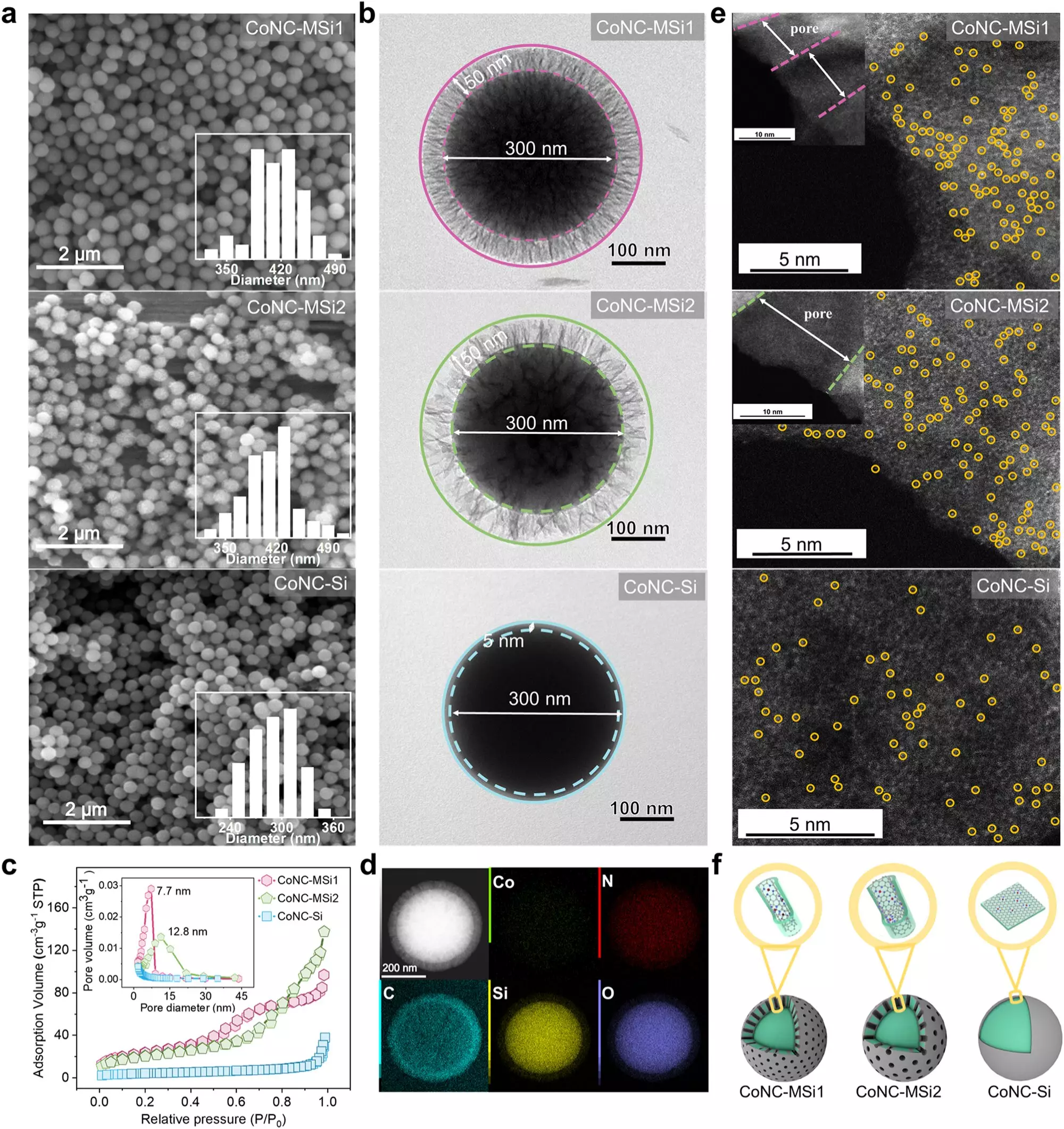
Although single-atom catalysts have emerged as powerful agents in chemical reactions, harnessing their full potential has been a challenge. The primary difficulties stem from the sluggish diffusion of reactants to the catalyst’s active sites and the excessive oxidants required for effective pollutant degradation. Historically, previous research had hinted that modifications to the physical confinement of these catalysts could yield efficiency gains through the concentration of pollutants and oxidants around the catalyst. However, the precise mechanics behind these improvements remained somewhat obscure, highlighting a gap in the existing body of knowledge.
The breakthrough discovery stemmed from a critical insight: by spatially confining SACs within minute nanometer-scale pores found in silica matrices, the research team dramatically enhanced both the speed and efficacy of the catalytic process. Their findings revealed that this configuration not only increased the local concentration of reactants but also fundamentally altered the catalytic reaction pathway. The team noted a shift from utilizing singlet oxygen, typically a reactive byproduct in many catalytic processes, to a direct electron transfer mechanism that allows for a remarkably more efficient degradation of pollutants.
The researchers reported an impressive 34.7-fold acceleration in the rate of pollutant breakdown when employing their newly developed SAC system compared to conventional methods. Additionally, the efficiency of oxidant utilization soared, climbing from 61.8% to an astounding 96.6%. This substantial leap illustrates not just a theoretical improvement but a practical one that could lead to transformative changes in water treatment methodologies. The system demonstrated versatility and robustness, effectively degrading a range of electron-rich phenolic compounds across varying environmental conditions, including real-world tests with lake water.
This enriching study not only sheds light on the operational dynamics of nanoconfined catalysts but also heralds an exciting future for low-carbon, efficient water purification technologies. By combining advancements in oxidation processes with the use of SACs, researchers open the door to significant potential developments in environmental management and pollution remediation. The implications of this work could extend beyond water treatment, providing a foundation for further innovations that address upcoming challenges in environmental science, promising a more sustainable approach to preserving our water resources.