Throughout history, the olfactory senses have held a unique place in human experience. The quest for enchanting aromas dates back to ancient civilizations, where fragrances were often linked to divine inspiration and personal well-being. This fascination with scent remains as relevant today, manifesting in the thriving perfume industry, which continues to evolve through innovation and creativity. Among the myriad scents that captivate our senses, ambrox stands out. Historically derived from ambergris, a rare byproduct of sperm whales, ambrox has secured its status as a highly sought-after fragrance across the globe.
Traditionally, the production of ambrox presented significant ethical and environmental concerns due to its dependence on the collection of whale-derived substances. While emerging technologies have shifted the focus towards sustainable alternatives, the extraction of ambrox from clary sage—a natural source—poses its challenges. The process is not only lengthy, requiring several intricate steps, but it also relies heavily on the availability of the plant itself, which can fluctuate dramatically. This scenario has catalyzed scientific inquiry into alternative methods for synthesizing ambrox in the laboratory, seeking to balance ecological responsibility with industrial demand.
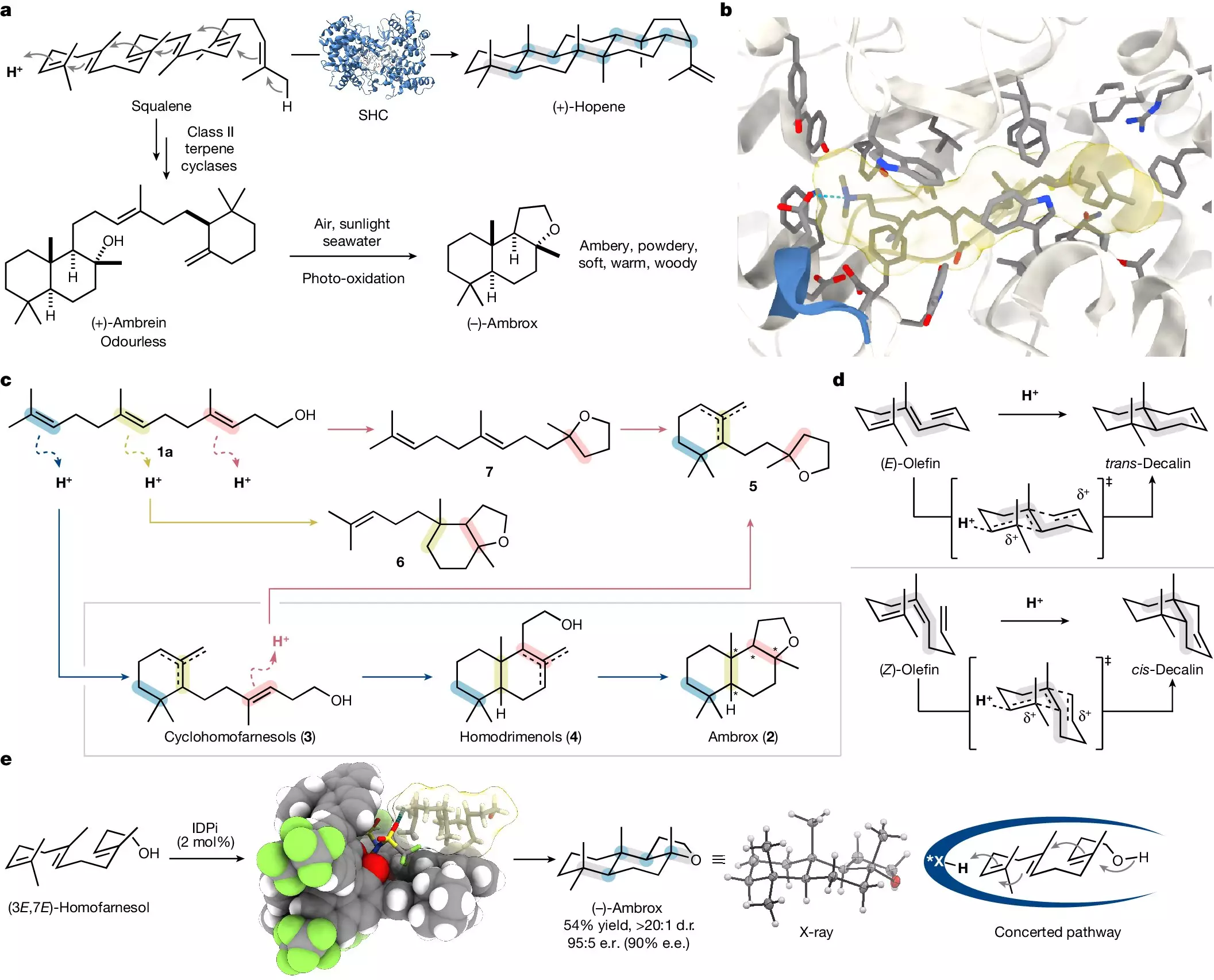
In a significant advancement, a team led by Prof. Benjamin List at the Max Planck Institute for Coal Research has developed a laboratory-based method to synthesize ambrox. Their findings, articulated in the journal Nature, emphasize an innovative approach that mirrors natural catalytic processes while making the procedure more efficient and sustainable. Central to their method is a complex reaction known as polyene cyclization, which enables the transformation of simpler organic compounds into intricate molecular structures in a single step. This remarkable efficiency stands in stark contrast to traditional methods that can take days to yield results.
Nature has long served as a template for scientific innovation. The scholars involved in this project were motivated by biological mechanisms and sought to replicate nature’s ability to form sophisticated molecules from basic inputs. According to Mathias Turberg, one of the doctoral students driving this research, capturing the essence of natural processes in a laboratory setting presents an exciting challenge for chemists. Dr. Na Luo, another lead contributor to the research, echoes this sentiment, expressing the aspiration of harmonizing laboratory techniques with nature’s inherent methodologies.
The synthesis process begins with nerolidol, a renewable C15 compound derived from various plant sources and amenable to large-scale production. Through collaborative efforts with BASF, this molecule is transformed into homofarnesol, which then undergoes a selective conversion to ambrox. The team utilized a specially designed catalyst combined with a fluorinated solvent that not only facilitates the reaction but also ensures the selectivity of the desired isomer—() ambrox—from 16 possible variants. This precision is crucial; the distinction between the isomers directly correlates with the fragrance’s quality and appeal.
The implications surrounding this research extend beyond academic curiosity. The newly developed process can be executed under milder conditions and accomplishes the transformation in just one night—a stark improvement compared to the standard biocatalytic methods that drag on for three to four days. Additionally, the ability to recover and reuse both the catalyst and solvent introduces a level of sustainability that could significantly affect future industrial applications.
As the research community continues to explore innovative methods for synthesizing complex natural products, the work of Prof. List and his team represents a pivotal moment in the realm of organic chemistry. Their work not only highlights the science’s capability to adapt and innovate but also reinforces the importance of ethical considerations in production processes. With promising results and scalable strategies in hand, the journey towards a more sustainable fragrance industry appears brighter than ever. Thus, the synthesis of ambrox stands as a testament to the synthesis between nature’s wisdom and human innovation, inviting further exploration of the tantalizing world of fragrance.