Ribosomes, often described as the cellular machinery for protein synthesis, play a pivotal role in the central dogma of molecular biology. They are responsible for translating messenger RNA (mRNA) into polypeptide chains, which then fold into functional proteins. Given their critical nature in biological systems, understanding the intricacies of ribosomal function has significant implications for biotechnology, medicine, and understanding diseases caused by protein misfolding.
A team of researchers at the University of Tsukuba has recently made strides in demystifying the inner workings of ribosomes by developing a novel computational model that simulates the ribosome’s internal environment. This project is groundbreaking, as it addresses the long-standing question of how protein structures begin to fold during their translation inside the ribosome tunnel—a narrow passage through which newly synthesized proteins traverse before being released. Prior to this research, the understanding of protein folding dynamics within ribosomes has remained incomplete, leaving scientists in search of clear mechanisms behind this critical biological process.
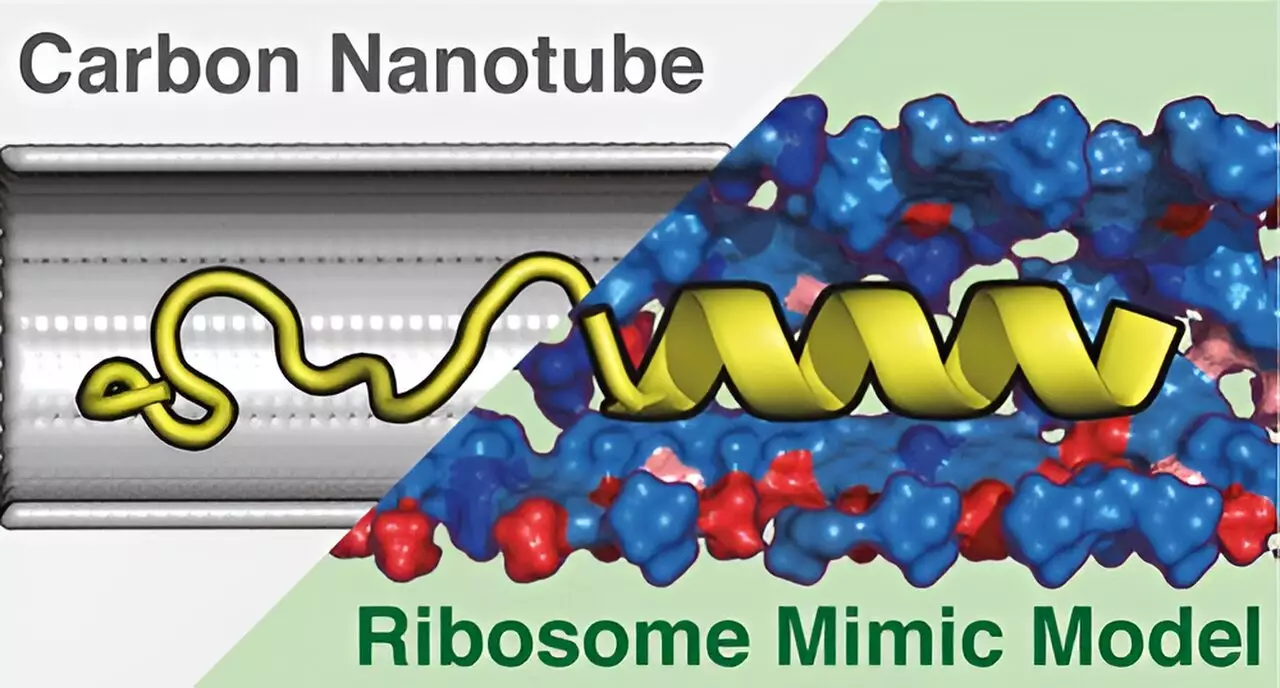
Through advanced computer simulations, the researchers meticulously investigated the chemical properties and structural dimensions of ribosome tunnels. They established the ribosome environment mimicking model (REMM), a cylindrical representation that not only mirrors the inner dimensions of ribosomes but also encompasses their unique chemical properties. This groundbreaking model sets a new benchmark in the field of molecular dynamics, allowing for a more accurate representation of protein structures that reflect real-world observations.
In contrast to the REMM, the researchers also explored a conventional carbon nanotube (CNT) model. While the CNT replicates the physical dimensions of the ribosome tunnel, it fails to account for the chemical complexity that is paramount for accurate protein modeling. This distinction proved crucial, as the findings showed that the REMM yielded results that were significantly more aligned with empirical data obtained from laboratory experiments.
The research highlights that the chemical diversity present in the REMM is an indispensable element influencing the protein-folding process. By imitating the ribosomal environment more faithfully than previous models, the REMM opens doors to a deeper comprehension of how proteins acquire their three-dimensional shapes during translation. Furthermore, this advancement lays the groundwork for subsequent studies focused on protein behavior in live cellular conditions.
The work produced by the researchers at Tsukuba University is not merely an academic exercise; it has profound implications in molecular biology, synthetic biology, and drug design. Advancements in the understanding of protein conformation can lead to enhanced therapeutic strategies for diseases associated with protein malfunctions, such as Alzheimer’s and Parkinson’s diseases. Additionally, as the REMM undergoes further refinements, its accuracy will likely lead to novel insights in genetic engineering and protein engineering, pushing the boundaries of current biotechnology applications.
The innovative study from Tsukuba University on ribosome simulation represents a significant leap forward in the field of protein synthesis research. By intertwining computational modeling with empirical findings, researchers are paving the way for a more comprehensive understanding of life’s fundamental processes.