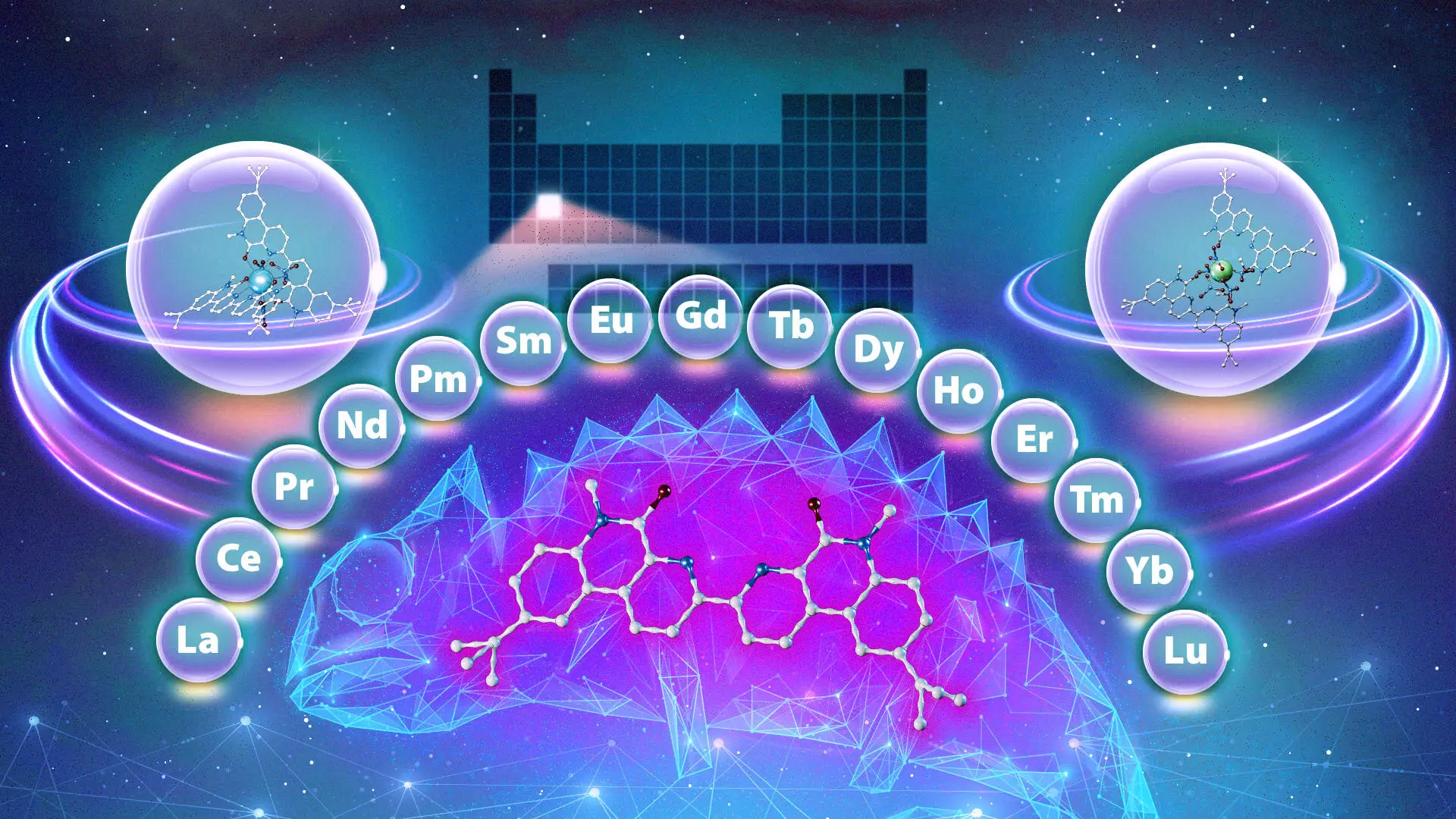
In the realm of modern technology, rare-earth metals—particularly the lanthanides—are indispensable. From the smartphones in our pockets to high-efficiency wind turbines, these metals play a crucial role in the development and functionality of various advanced materials. Despite their classification as “rare,” many of these elements are relatively abundant in the Earth’s crust but are challenging to extract and purify. Recent research at Oak Ridge National Laboratory (ORNL) has unveiled a potential breakthrough in the extraction process, showcasing a novel ligand that may revolutionize how we approach the purification of these vital resources.
The lanthanides, a series of 15 elements, exhibit stunning chemical properties that render them vital for numerous applications, including medical devices and electronics. Their chemical similarities, however, pose significant challenges in the extraction process. Each lanthanide is only slightly different in size and charge, making selective separation a complex task. Subhamay Pramanik, a former postdoctoral researcher at ORNL, highlighted the difficulty in isolating these metals due to their closely related characteristics. Traditional methods of separation remain a costly and environmentally taxing endeavor.
Current industrial practices often rely on multi-step processes to extract lanthanides in a specified order, typically from heavy to light or vice versa. This systemic approach, while effective, generates considerable waste and suffers from inefficiencies that ultimately drive up costs. Thus, there is a pressing need for innovative methods that reduce the environmental impact and enhance the efficiency of this extraction process.
The turning point in this landscape may lie in the discovery of a new ligand that behaves in a manner reminiscent of a chameleon. ORNL researchers, in collaboration with Vanderbilt University, identified that this ligand can adapt its binding preferences based on external conditions, such as the acid concentration in the solution. Remarkably, the ligand’s ability to switch between binding light, heavy, and mid-weight lanthanides is groundbreaking. This dynamic behavior not only simplifies the extraction process but also minimizes the number of steps involved.
Santa Jansone-Popova, an ORNL scientist, emphasized that most ligands display a preference for either lighter or heavier lanthanides. In contrast, the newfound chameleon ligand demonstrates versatility, potentially allowing for the separation of different lanthanides within a single interaction. This attribute creates a promising avenue for enhancing efficiency in refining methods, a critical consideration as demand for these materials continues to increase.
Understanding the mechanisms that underlie this unique behavior is as critical as the discovery itself. The research explains that the ligand’s binding characteristics vary with time and environmental factors, leading to different outcomes in separation processes. This insight not only lays the groundwork for further exploration of chameleon-like ligands but also invites investigation into the broader implications of adjustable chemical behaviors in materials science. Ilja Popovs, an ORNL co-leader on the study, noted that structural similarities among ligands do not guarantee that they will behave identically. This new understanding propels the field toward discovering more compounds that could exhibit similar adaptable characteristics.
The ramifications of the chameleon ligand’s capabilities extend beyond academic curiosity; they reach into the realms of industry and environmental sustainability. By taking advantage of a ligand that reduces the steps involved in rare-earth metal separation, industries could not only lower costs but also mitigate the ecological footprint associated with rare-earth extraction methodologies. The prospect of minimizing waste and increasing the efficiency of resource recovery is timely, especially in light of global efforts to push towards sustainable technology solutions.
Furthermore, these findings can spur ongoing research efforts aimed at synthesizing additional ligands with adjustable properties. As the demands for rare-earth metals rise, the need for efficient, cost-effective, and environmentally friendly extraction methods will only become more urgent.
The discovery of the chameleon ligand represents a significant advancement in the field of chemistry with profound implications for extracting and purifying rare-earth metals. Through innovative research, scientists have not only unlocked new potential for separating lanthanides but have also set in motion a pivotal shift towards more sustainable practices in the industry. As further investigations unfold, the possibilities to enhance our approach to these essential materials seem boundless, paving the way for greener technologies and a better understanding of chemical behaviors. The humble chameleon, it turns out, may hold the key to one of the most pressing challenges in the realms of modern science and technology.