In the landscape of organic chemistry, Z-alkenes present themselves as a cornerstone for a variety of reactions and compounds. Defined by the unique placement of substituents on the same side of a carbon-carbon double bond, Z-alkenes are not just limited to academic curiosity; they are extensively used in biological systems and industrial applications. However, synthesizing these compounds has traditionally posed significant challenges, particularly when conventional thermodynamic techniques fall short. The limitations of these methods underscore a pressing need for innovative solutions, drawing attention to the promising approach of photoisomerization.
At its core, photoisomerization involves the transformation of one isomer into another through light absorption. This captivating process serves as a beacon of hope for chemists grappling with the restrictive pathways to Z-alkenes. By shifting focus to the photoisomerization of E-alkenes—its structural counterpart—researchers have uncovered a wealth of possibilities. The process itself hinges on harnessing light energy to convert E to Z configurations effectively and efficiently.
The existing literature is peppered with various methodologies to facilitate this intriguing chemical conversion. Among these, a standout method involves the use of continuous flow systems, where immobilized photosensitizers in ionic liquids promise enhanced yields. However, practitioners have noted the cumbersome nature of these methods, especially when trying to incorporate sophisticated techniques such as high-performance liquid chromatography (HPLC) into the process.
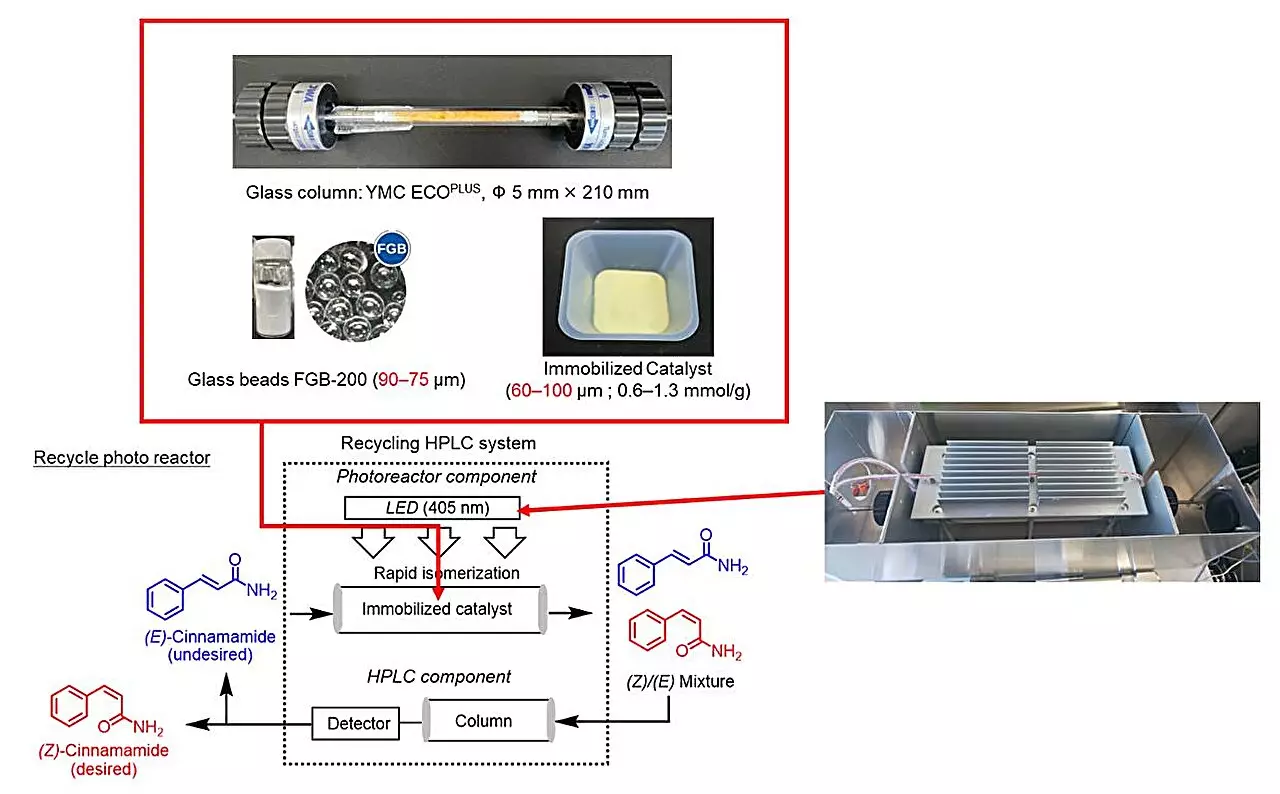
In a groundbreaking study led by Professor Hideyo Takahashi and his team from Tokyo University of Science, the conventional barriers facing photoisomerization were challenged. Their research, recently published in The Journal of Organic Chemistry, employed not just any means of photoisomerization but utilized a state-of-the-art recycling photoreactor in conjunction with HPLC technology.
The innovative aspect of this research lies in its genesis: initially aimed at deracemization, the method was repurposed to convert E-cinnamamides into Z-cinnamamides. The structured loop of their photoreactor serves to continuously recycle elements within the reaction, promoting efficiency and sustainability—a magnificent example of recycling principles applied to chemical processes.
Central to the success of this innovative approach is the choice of photosensitizers, compounds that absorb light and enable the reaction to proceed. Takahashi’s team meticulously screened various candidates, ultimately identifying thioxanthone as the most effective. Their method of immobilizing thioxanthone on modified silica gel stood out, resulting in increased catalytic activity and preventing loss of the sensitizer during the process.
Interestingly, this solid-phase immobilization challenged the typical notion that solid reactions are inherently slower, illustrating how the interaction of functional groups can considerably enhance reaction rates. This research showcases not just the adaptability of photosensitizers but emphasizes the importance of the right conditions for catalysis in organic synthesis.
Beyond the technical advancements, the implications of this research ripple through the fabric of sustainable chemistry. By transitioning to a closed-loop recycling system, the team’s findings contribute to a significantly greener approach to producing Z-alkenes. This advancement signals a pivotal shift towards environmentally-conscious methodologies in pharmaceuticals that prioritize sustainability without compromising on effectiveness.
Furthermore, the development of Z-alkenes through this innovative system can inspire similar approaches across various sectors within organic and medicinal chemistry. The commitment to harnessing light-driven processes can redefine synthetic pathways, ultimately leading to a transformative impact on how we produce essential compounds in a world increasingly aware of its ecological footprint.
In an era where sustainability must be at the forefront of scientific endeavors, Takahashi’s pioneering work offers a glimmer of hope, demonstrating that innovative chemistry can pave the way for a brighter, more eco-friendly future. This study opens new avenues not only for research but also practical applications that prioritize both efficiency and environmental considerations.