As the world grapples with the urgent need for sustainable practices to mitigate climate change, innovative strategies for carbon dioxide (CO₂) reduction have emerged as a focal point for scientific research. The transformation of CO₂ into valuable products not only addresses excess emissions but also promotes a circular economy. However, achieving high selectivity in these reduction processes remains a significant hurdle. Researchers at the University of Twente, under the guidance of Georgios Katsoukis, have made notable advancements in understanding how the surrounding chemical environment impacts the effectiveness of this conversion, specifically when using copper electrodes.
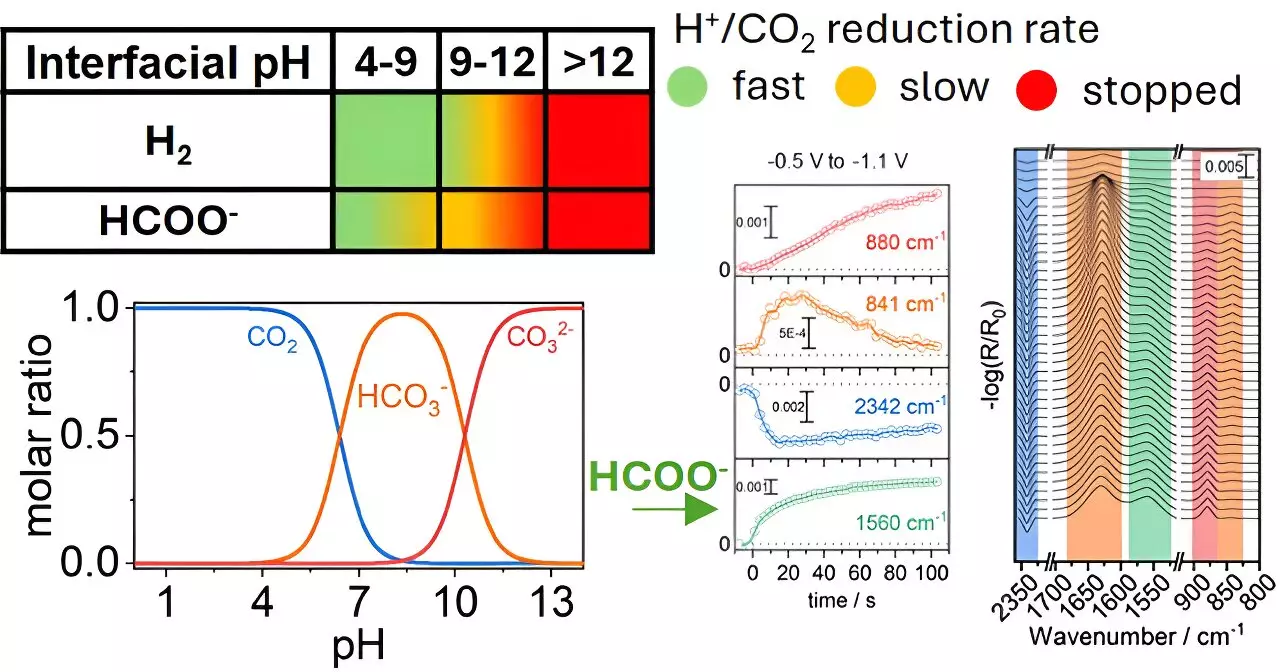
The latest findings, published in the journal ACS Catalysis, reveal that the chemical reactions involving CO₂ at copper electrode surfaces are highly sensitive to their local environment, particularly pH levels. The research team conducted experiments to illustrate that modifying the pH around these electrodes can significantly enhance the efficiency and speed of the conversion of CO₂ into formate—a compound with a host of industrial applications. This pivotal discovery challenges the previously held notion that the material of the catalyst itself was the sole factor influencing reaction outcomes. Instead, the results emphasize that attention must also focus on the chemical conditions in which these reactions take place.
This refined perspective on catalyst efficiency introduces a new layer to the understanding of CO₂ reduction reactions. Historically, scientists have concentrated on improving the intrinsic properties of catalyst materials. The findings from this research suggest that by fine-tuning the surrounding environment—such as altering pH levels—researchers can not only enhance product selectivity but potentially prolong the lifespan of the electrodes used in these reactions. This signifies a paradigm shift toward a more holistic view of catalytic processes that integrates both material characteristics and environmental conditions.
The implications of this research extend beyond laboratory settings, potentially shaping future CO₂ reduction technologies. By strategically manipulating the chemical environment, the efficiency of systems designed for converting CO₂ into valuable resources could markedly improve. This is crucial for realizing more effective solutions to combat climate change. The innovative focus on optimizing not just the catalyst but also the reactions’ surrounding conditions lays a solid foundation for subsequent explorations in this area.
The research from the University of Twente serves as a critical reminder of the interconnectivity between materials science and chemical processes when addressing global challenges such as CO₂ emissions. By fostering tailored environments for chemical reactions, scientists can pave the way for more selective, efficient, and sustainable CO₂ reduction technologies. This research not only holds promise for industrial improvements but stands as a wise investment in the transition toward a more sustainable and circular economy. In essence, by embracing a multifaceted approach that considers both catalysts and their surrounding conditions, we can move closer to practical, impactful solutions for one of our planet’s most pressing issues.