The concept of self-assembly in biology mirrors the dream of a DIY enthusiast who hopes for seamless construction—where individual parts meld into a cohesive entity without the need for manual input. Essentially, this phenomenon is the cornerstone of many biological structures, including proteins, lipids, and even entire viruses. Knowing how these complex organizations come to life can provide insights not only into the fundamentals of life but also into the burgeoning field of supramolecular chemistry.
Supramolecular chemistry deals with the arrangement and interactions of molecules and has garnered attention for its potential to innovate material science. Researchers are experimenting with various methods to assemble large molecular structures by manipulating the forces that hold smaller units together. For instance, the integration of polymers through specific interactions allows scientists to engineer “smart materials” that can adapt to environmental stimuli—for example, responding to changes in temperature or chemical presence. Nevertheless, there remains a significant gap in understanding the dynamics and potential applications of these self-assembling systems.
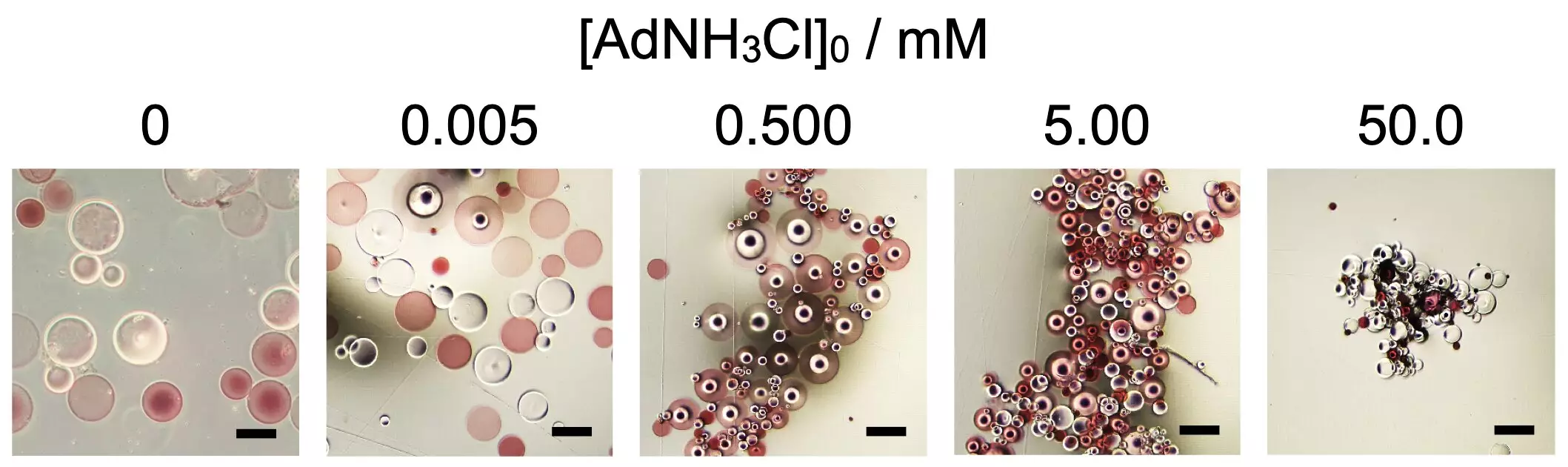
In a noteworthy study led by researchers from Osaka University, the self-assembly of spherical microparticles derived from poly(sodium acrylate), a superabsorbent polymer, was investigated. The research provided new insights into how additives can significantly influence the formation and shape of these microparticles. By introducing 1-adamantanamine hydrochloride (AdNH3Cl) at a precise concentration, the study revealed a critical point at which the self-assembly process could be initiated. Adding elements such as β-cyclodextrin (βCD) and adamantane (Ad) residues to the polymer chain allowed researchers to fine-tune the assembly properties and resultant forms.
The study emphasized the parallels between these synthetic microparticles and biological proteins, which consist of polymers formed from amino acids. Similar to how hydrogen bonds and electrostatic forces govern protein folding, the interactions among these tailored microparticles can dictate their ultimate shape. The lead researcher, Akihito Hashidzume, posited that understanding these complex interactions in artificial systems can illuminate our grasp of biological processes—where organisms are, in essence, intricate networks of supramolecular structures.
The implications of the Osaka University study are immense. By further dissecting how macroscopic assemblies can be controlled through microscopic interactions, researchers can not only advance material science but also open doors to innovative active materials that react and adapt in real-time. Insights gained from this research are poised to inform future explorations into biological systems—potentially unlocking secrets of organismal shapes and functions. As Akira Harada, senior author of the study highlighted, this knowledge could provide fertile ground for preventing issues related to material stability and exploring adaptive technologies.
Understanding the principles governing self-assembly—whether in an artificial laboratory or nature—could lead to revolutionary advancements in materials science. By looking at how small-scale interactions lead to large-scale structures, the field of supramolecular chemistry stands on the precipice of exciting discoveries, enhancing our comprehension of the natural world as well as our ability to manipulate it.