In the intricate dance of life, one of the defining characteristics is the self-organization of matter, a phenomenon that continuously puzzles scientists. What appears lifeless can, under certain conditions, organize itself into structures that resemble living entities. At the core of these investigations lies an intriguing question: How does this self-organization occur, particularly in the context of cellular processes? Recent research led by Professor Anđela Šarić and her team at the Institute of Science and Technology Austria (ISTA) sheds light on a previously unexplored mechanism essential for bacterial cell division. Dubbed “dying to align,” this mechanism reveals how misaligned filaments within bacterial cells can spontaneously disassemble and reorganize, ultimately fostering the formation of a vital structure necessary for cell division.
Revisiting the Role of FtsZ in Cell Division
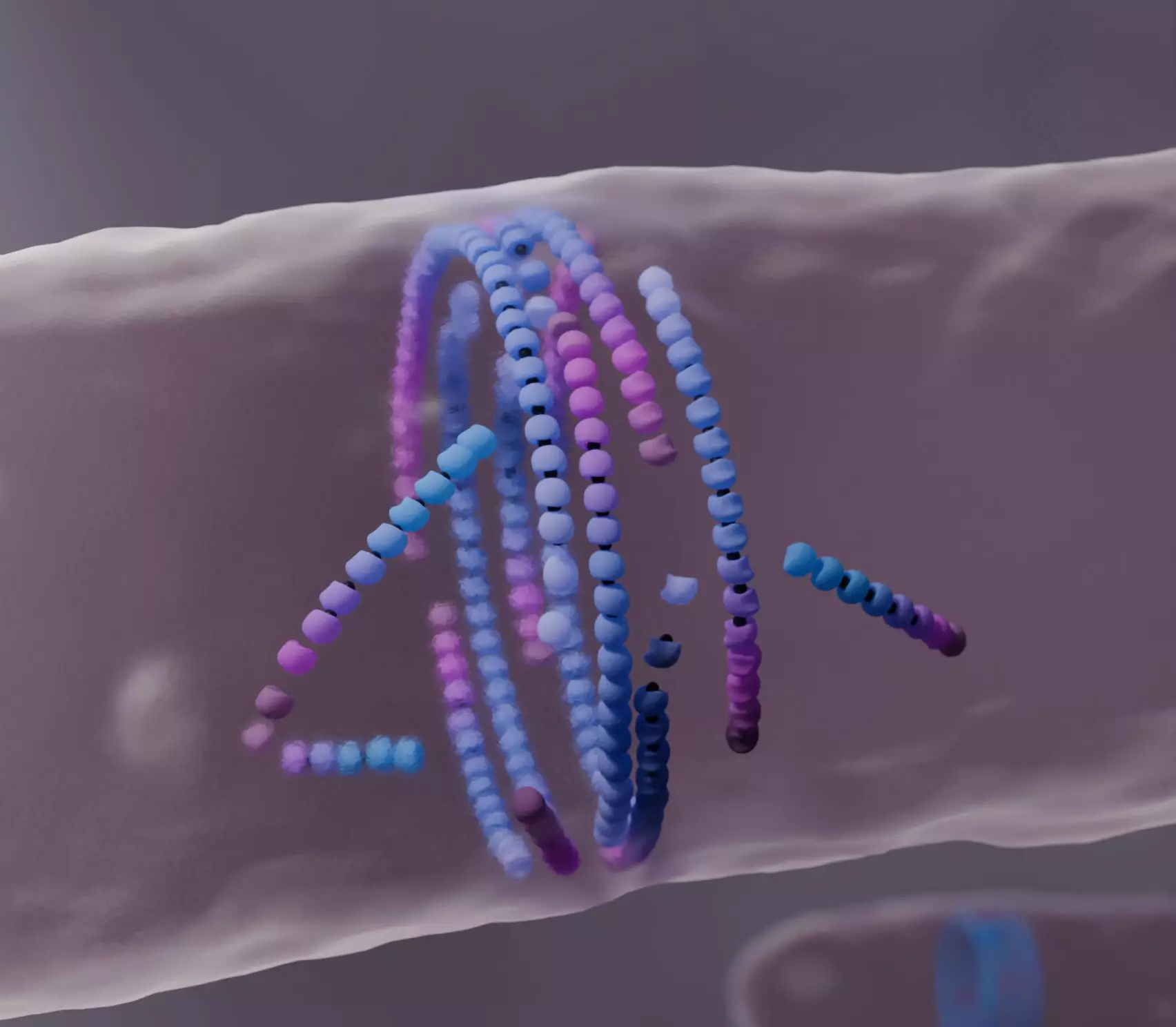
The protein FtsZ plays a crucial role in the process of bacterial cell division. The significance of this protein is underscored by its ability to self-assemble into a ring-like structure known as the bacterial division ring. This ring is not merely a static formation; it actively contributes to the synthesis of a new cell wall, thereby segregating the two daughter cells during division. Although the functional importance of FtsZ has long been recognized, the specific physical mechanisms governing its assembly have remained elusive. In their pioneering study published in Nature Physics, the Šarić group aimed to bridge this gap by modeling the dynamic interactions of FtsZ and how these interactions lead to cohesive self-organization.
A Insights-Based Computational Model
Using advanced computational techniques, the researchers created a model to simulate the behavior of FtsZ filaments during cell division. The novel aspect of their research focused on misaligned filaments, which tend to encounter obstacles during their assembly process. Unlike traditional models, which present a simplified view of filament dynamics, the researchers discovered that misaligned filaments exhibit a unique behavior: they “die” upon collision with obstacles. This dissolution isn’t a random event; instead, it represents a systematic part of a feedback loop that enhances the overall organization of the filament structure. By reorganizing and aligning themselves, the remaining filaments facilitate the correct formation of the bacterial division ring.
The term “dying to align” poignantly encapsulates the findings of the study. As misaligned FtsZ filaments deteriorate, their death aids in refining the configuration of the surviving filaments, thereby enhancing the formation of well-aligned structures critical for effective cell division. This observation is not merely a footnote in the narrative of self-organization; it suggests a transformative perspective on how active matter, which typically includes various biological and synthetic materials, can adaptively respond to environmental cues. Consequently, this research points towards emerging applications in materials science, particularly in the development of self-healing synthetic materials that mimic this biological process.
Experimental Validation and Collaborative Synergy
The collaboration between the Šarić group and experimental teams from the University of Warwick and ISTA was pivotal in validating the computational findings. During a multi-disciplinary conference, Šarić’s team encountered Dr. Séamus Holden, who was pioneering live imaging techniques to visualize FtsZ ring formation in bacterial cells. Their discussions led to a fascinating alignment of experimental findings with the computational predictions of the Šarić group. This cross-validation not only solidified the conclusions drawn from computational models but also underscored the power of collaborative research in unraveling complex biological phenomena.
As the research unfolds, the implications extend beyond understanding bacterial cell division. The principles uncovered could catalyze advancements in synthetic biology and materials science. The investigation into FtsZ’s unique turnover-driven self-organization challenges conventional perspectives on active matter. It encourages researchers to rethink existing paradigms surrounding molecular interactions and self-assembly, potentially leading to groundbreaking discoveries in creating lifelike, self-repairing materials.
The insights into how bacteria manage to align their internal structures through a process of self-dissolution and reassembly are not just a scientific curiosity—they open doors to a deeper understanding of life itself and present opportunities to engineer materials that could one day mimic these extraordinary self-healing capabilities. As the Šarić group continues to explore these fascinating dynamics, we might be inching closer to bridging the gap between the non-living and the living, ultimately redefining our comprehension of matter itself.