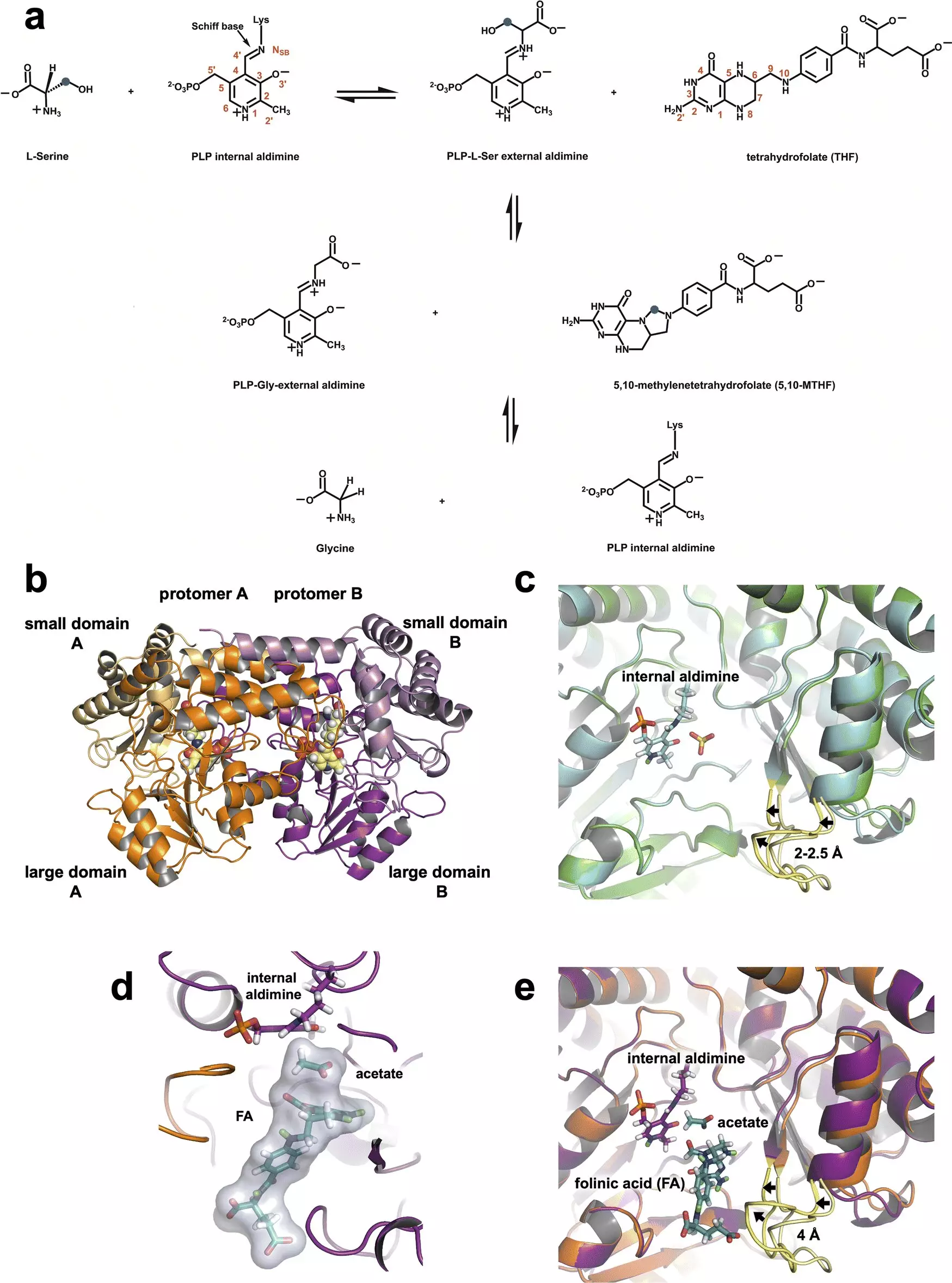
Recent neutron experiments have unveiled critical insights into the enzymatic mechanisms that play a pivotal role in cancer metabolism. Conducted at the Department of Energy’s Oak Ridge National Laboratory (ORNL), these studies spotlight serine hydroxymethyltransferase (SHMT), an enzyme crucial for cell division. Utilizing neutron diffraction techniques at the Spallation Neutron Source and the High Flux Isotope Reactor, the research team has successfully mapped the minute atomic interactions that underlie SHMT’s function. Understanding these interactions is essential for the development of precise inhibitors that could disrupt cancer’s unchecked proliferation.
The role of SHMT in cellular metabolism is particularly fascinating. Cancer cells exhibit a tendency to hijack metabolic pathways, converting normal enzymatic activities into mechanisms that promote rapid division and growth. The insights gleaned from identifying SHMT’s atomic structure highlight the enzyme’s potential as an intervention point in cancer treatment.
The Importance of Neutron Diffraction in Structural Biology
Neutron diffraction has emerged as a vital tool in structural biology, particularly in revealing the roles of light atoms like hydrogen in biological molecules. In the case of SHMT, the research identified a key amino acid residue, glutamate, vital to the enzyme’s catalytic activity. This amino acid behaves both as an acid and a base, providing a unique dual-functionality that has been a matter of speculation since the early 1980s. By using neutrons to visualize the proton’s location on glutamate, scientists are better able to understand the enzyme’s reaction mechanisms.
Co-author Robert Phillips emphasized that neutron data confirmed the unexpected behavior of glutamate, which retains its proton instead of losing it to participate in reactions as anticipated. This refined understanding allows for greater precision in targeting SHMT with drug design strategies, paving the way for novel therapeutic approaches.
SHMT is pivotal in a biochemical pathway known as one-carbon metabolism, which occurs in mitochondria, the powerhouse of the cell. It facilitates the conversion of the amino acid serine into glycine, crucial for synthesizing nucleic acids—key components of DNA and RNA. These processes are fundamental to cell division and proliferation, especially in the context of cancerous cells, which often exhibit elevated levels of SHMT to fuel their accelerated division.
The research team previously combined neutron and X-ray crystallography to explore SHMT’s structure before its interaction with tetrahydrofolate, a critical substrate in this metabolic pathway. Recently, they captured a snapshot of SHMT engaged in subsequent reactions, achieving a previously elusive understanding of how this enzyme operates at a fundamental level.
The identification of the roles played by hydrogen atoms within SHMT offers promising avenues for drug design aimed at interrupting its activity. By stopping SHMT’s interaction with substrates like tetrahydrofolate, researchers can strategically thwart the metabolic processes that enable cancer cell proliferation.
The research signifies a major step forward in the quest for effective cancer therapies. Unlike existing drugs that target various enzymes later in the metabolic pathway, SHMT presents an earlier intervention point. This approach could potentially mitigate the cancerous growth before it escalates, enhancing the efficacy of treatment regimes.
Challenges in Cancer Treatment and Future Directions
Despite these advancements, the unique nature of cancer pharmacology poses significant challenges. Unlike infectious diseases where external pathogens can be targeted, cancer treatment often involves attacking the body’s own cells. This duality results in complex side effects for patients undergoing chemotherapy. The goal is to craft inhibitors that can selectively target cancerous cells while sparing healthy tissues.
The researchers’ findings highlight the crucial need for precision in drug design, and ongoing developments at ORNL signify a shift towards using advanced technologies, such as artificial intelligence, to aid in the rapid development of tailored therapies. As noted by William Nelson from Johns Hopkins University, the integration of gene sequencing and protein structure prediction can revolutionize the pace at which new drugs are created.
Moving forward, collaborative research combining neutron and X-ray diffraction at facilities such as ORNL and Argonne National Laboratory will be essential in tackling the complexities of cancer treatment. Enhanced neutron production capabilities promise ever-shorter data collection times, facilitating quicker iterations in drug design. The ultimate aim is to develop small-molecule inhibitors capable of disrupting critical metabolic pathways in cancer cells, thus improving patient outcomes and reducing treatment-related side effects.
This exploration into SHMT and its implications for cancer therapy underscores the importance of fundamental research in the larger framework of medical innovation. By understanding the intricate biochemistry of enzymes, researchers hope to unlock new methods for combatting one of the world’s most daunting health challenges—cancer.