The alarming rise in carbon dioxide (CO2) emissions has underscored the urgent need to address climate change, which poses a significant threat to ecosystems and human society alike. Among various strategies to combat this crisis, cement—an essential component in construction—has emerged as a potential ally in capturing CO2. The process known as carbonation entails the conversion of CO2 into stable mineral forms, specifically calcium carbonate. By understanding and enhancing this phenomenon, researchers aspire to turn cement into a tool for climate action rather than an aggravator of greenhouse gas emissions.
At the core of cement carbonation lies the interaction between CO2 and calcium silicate hydrates (C–S–H), which are produced during cement hydration. During the carbonation process, CO2 gets dissolved in the pore water of cement and subsequently reacts with C–S–H to form carbonate ions. These ions then interact with calcium ions, precipitating calcium carbonate. Despite its promising potential, the intricacies of carbonation mechanisms remain poorly understood due to the dynamic and unstable nature of cement paste components.
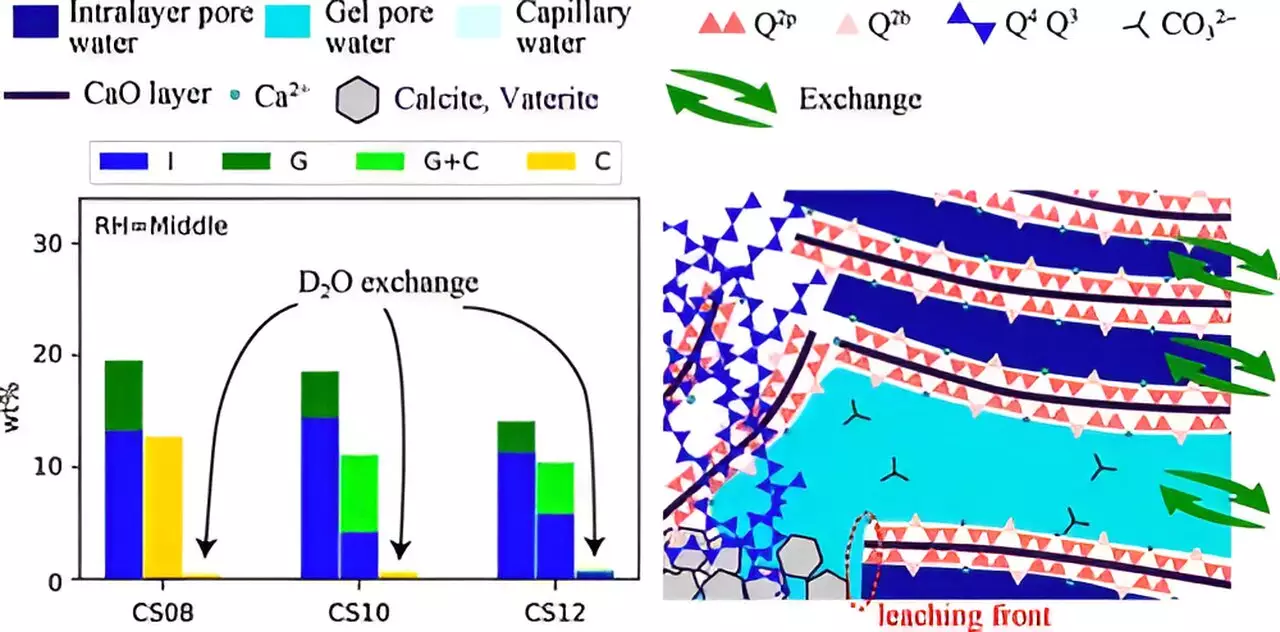
Researchers have identified several factors affecting the efficiency of carbonation, such as relative humidity (RH), the solubility of CO2, and the calcium-to-silicate (Ca/Si) ratio. Moreover, the role of ionic transport and pore structure within the C–S–H matrix is crucial for comprehending how carbonation progresses. Understanding these complexities is critical for optimizing the reactive properties of cement materials and enhancing their carbon-capturing capabilities.
A pioneering study published in The Journal of Physical Chemistry C showcased the groundbreaking work of Associate Professor Takahiro Ohkubo and his interdisciplinary team. By employing advanced techniques like 29Si nuclear magnetic resonance (NMR) and 1H NMR relaxometry, the researchers explored the interplay between water transport and structural modifications during carbonation under various conditions. These methods allowed them to observe water dynamics and the resultant changes in the C–S–H matrix, offering new insights into the carbonation process.
To simulate real-world carbonation, the researchers subjected synthesized C–S–H samples to an accelerated carbonation environment with an atmosphere rich in CO2. This brief duration experiment, although differing from the natural timeframe of decades that typical carbonation entails, provided profound insights into the reaction mechanisms at play.
The outcomes of the study revealed that carbonation is influenced significantly by the Ca/Si ratio and RH conditions. Notably, lower RH levels, combined with a higher Ca/Si ratio, tended to create smaller pores in the C–S–H structure. This pore size reduction hindered the leaching process of calcium ions and water, culminating in ineffective carbonation. Such findings underscore the necessity of considering both structural changes and mass transport dynamics in efforts to enhance carbonation efficiency.
Associate Professor Ohkubo emphasized the importance of this dual focus, stating, “Understanding the combination of structural modifications and mass transfer is vital for improving the carbonation process.” This perspective marks a shift from previous research that largely concentrated on structural changes in isolation.
Beyond its implications for cement and construction, the relevance of carbonation extends to natural processes as well. Organic materials undergo similar carbonation reactions, providing a broader context for understanding carbon capture in both built and natural environments. The research conducted by Ohkubo and his team has the potential to inspire the development of innovative building materials capable of sequestering atmospheric CO2 effectively.
Consequently, this new understanding of carbonation mechanisms could pave the way for novel strategies in sustainability and environmental engineering. As the construction industry evolves, embracing such materials may contribute significantly to mitigating climate change, aligning economic growth with ecological health.
This transformative study sheds light on the fundamental processes governing carbonation in cement-based materials, highlighting avenues for future research and applications. As the world grapples with the pressing need for sustainable practices, integrating carbon capture into the very fabric of building materials represents an innovative approach to tackle the climate crisis. By unlocking the potential of carbonation, we may reshape not only the construction landscape but also contribute meaningfully to the reduction of atmospheric CO2, reinforcing the critical interplay between industry and sustainability. The path forward demands collaboration and innovation, with the ultimate goal of fostering a more sustainable built environment capable of absorbing the very emissions it produces.