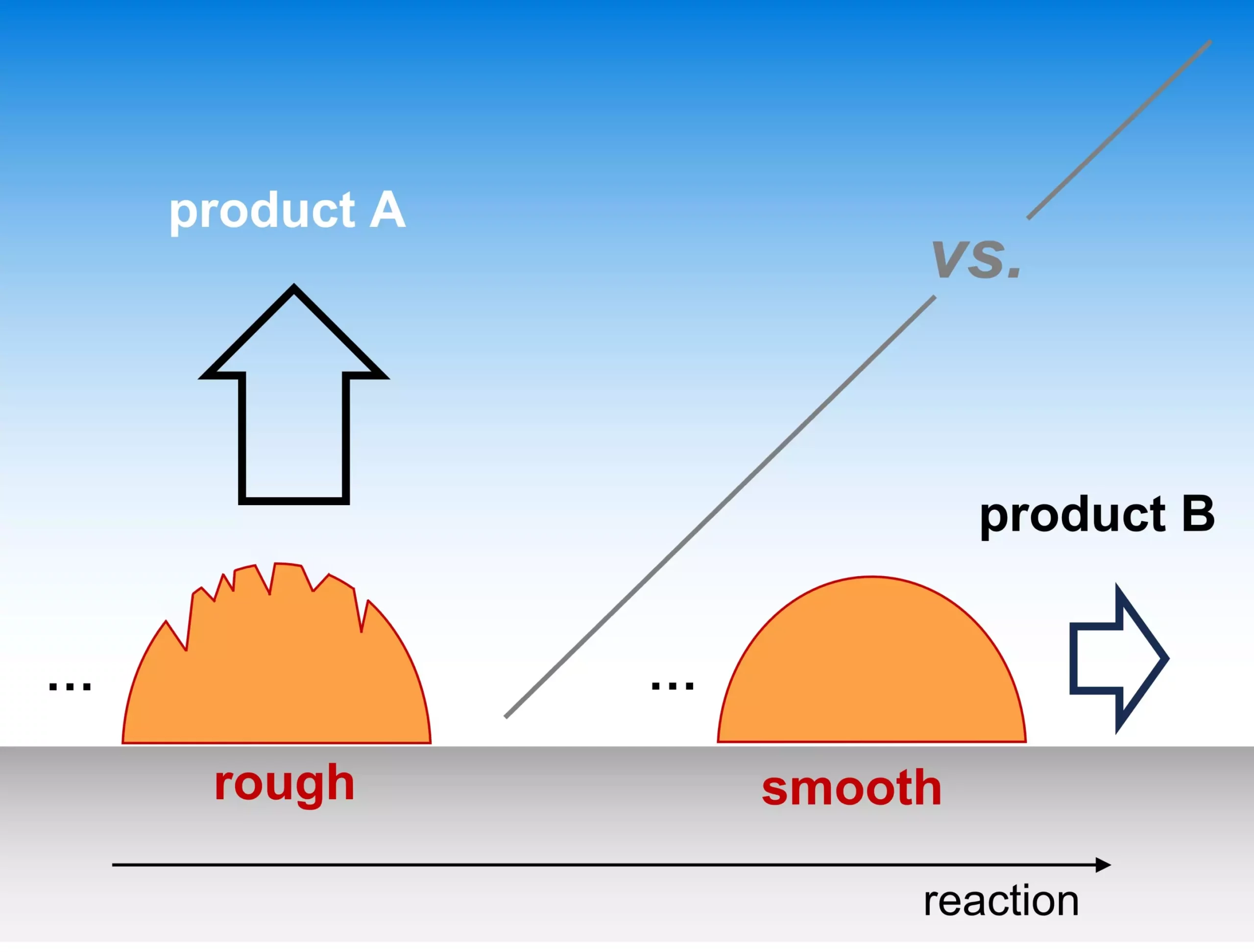
The recent findings from the Fritz Haber Institute’s Theory Department cast new light on a pivotal factor in electrocatalytic reactions: the morphology of catalysts. Highlighted in their publication in Nature Catalysis, this groundbreaking analysis reveals how the roughness of a catalyst’s surface can significantly influence the product outcomes of critical reactions, such as the transformation of CO2 into renewable fuels and the vital generation of water in fuel cells. This insight challenges the prevailing notion that emphasizes the atomic characteristics of active sites, prompting a reevaluation of design methods for catalysts in electrochemical applications.
A Focus Shift to Surface Morphology
What is particularly striking about the study is its emphasis on catalyst roughness—a characteristic often overshadowed by atomic structuring in discussions of catalytic effectiveness. The research indicates that variations in surface texture can lead to divergent reaction pathways. When considering electrocatalysis, where the aim is to drive chemical processes via renewable electricity, understanding the impact of morphology on selectivity opens up a vast landscape of potential optimization avenues. To think that the surface’s texture can make such a substantial difference is revolutionary, as it suggests that refining catalyst design is as much about the macroscopic features as it is about the molecular composition at play.
Electrocatalysis: The Path to Sustainability
In today’s world, where the pursuit of sustainable energy solutions is more critical than ever, heterogeneous electrocatalysis stands out as a promising technology. This approach not only addresses the need for carbon-neutral energy production but demonstrates a capacity to transform how we generate fuels and chemicals under mild conditions effectively. The mechanisms driving these transformations, powered by charge transfers at solid-liquid interfaces, rely heavily on the efficiency of catalytic surfaces. Therefore, the new focus on roughness can help shift the paradigm towards achieving cleaner energy more efficiently.
The Role of Kinetic Modeling
One of the groundbreaking aspects of the Fritz Haber research is the introduction of a multi-scale kinetic model that quantifies how selectivity is influenced by both the transport rates of reactants within the electrolyte and the density of active sites on the catalyst. This model not only simplifies the complexities of reaction pathways but also establishes a correlation between the roughness of the catalyst and its effectiveness in driving selectivity. The ability to predict chemical behavior through this model underscores its utility in the deductive reasoning needed for ongoing catalyst development and optimization.
A New Dawn for Catalyst Research
As the research underscores the importance of catalyst morphology across various scales, it opens avenues for a more nuanced understanding of electrocatalytic processes. This perspective not only enhances our fundamental grasp of reaction mechanisms but also posits new strategies to enhance the selectivity and longevity of catalysts in industrial applications. This study serves as a clarion call for researchers and industry practitioners alike to adopt a broader scope when it comes to catalyst design—one that encompasses both microscopic structuring and the macroscopic features of these crucial materials. By pivoting to focus on morphology, we may unlock new frontiers in sustainable energy technologies, providing a vital lifeline in our quest for a greener future.