In the realm of organic chemistry, innovation often paves the way for breakthroughs that could significantly impact drug discovery and development. A recent study from researchers at Tokyo Tech has unveiled an innovative approach to synthesizing 2D/3D fused frameworks utilizing inexpensive quinolines. This methodology not only showcases the versatility of quinolines but also presents a cost-effective solution for crafting complex organic molecules with significant medical applications. The implications of this research extend far beyond a singular synthesis method; they herald a new era in customized drug candidate development.
The Quinolines Advantage
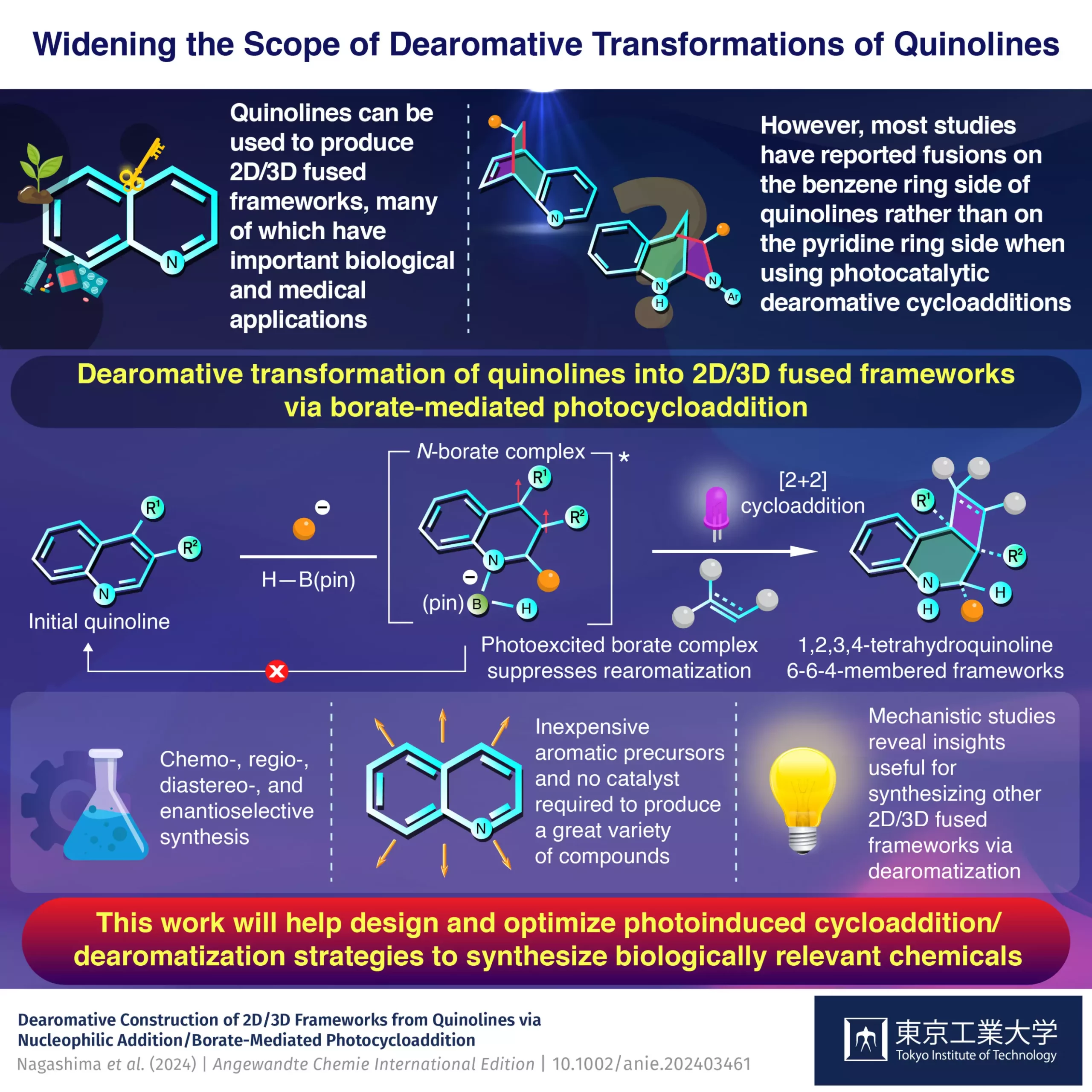
Quinolines have captivated the interest of chemists for a good reason. Their unique chemical structure, which consists of an electron-rich benzene ring fused to an electron-deficient pyridine ring, allows for tailored modifications. This unique electronic configuration is crucial because it provides a robust framework for synthesizing a wide array of diverse compounds. The ability to separately modify each ring by manipulating reaction conditions transforms quinolines into an ideal feedstock for drug development. This study takes an important step toward unlocking the full potential of quinolines, which have been historically underutilized.
Dearomative Photocycloadditions: A Game Changer
At the core of this newly revealed synthesis strategy is the process of dearomative photocycloaddition. Previously focused primarily on attaching reactants to the benzene side of quinoline, researchers had not fully harnessed the potential of the pyridine side. The Tokyo Tech team innovatively redirected attention to the pyridine side by utilizing a light-sensitive borate intermediate. Their work demonstrates that leveraging a compound known as pinacolborane, or H–B(pin), serves as an effective catalyst for facilitating reactions exclusively on the pyridine side. This strategy not only broadens the applications of quinolines but also invites a reconsideration of the classical methods that have dominated organic synthesis.
The Mechanistic Insights
Understanding the underlying mechanisms of chemical reactions is vital for advancing synthetic methods. The Tokyo Tech researchers conducted a series of rigorous experiments and theoretical analyses, unveiling a precise pathway through which quinoline reacts with an organolithium compound before forming a borate complex. Such mechanistic clarity is crucial, as it enables chemists to manipulate reactions with greater precision and predictability. Notably, the photoexcited borate complex enhances the efficiency of the cycloaddition process by suppressing rearomatization—a common pitfall in traditional methods—thus minimizing unwanted byproducts.
Efficiency Meets Versatility
One of the standout features of this methodology is its efficiency. The traditional synthetic routes often require extensive reaction times and numerous steps, making them both cumbersome and costly. In contrast, the Tokyo Tech approach significantly reduces these requirements. Notably, this methodology does not rely on catalysts, further diminishing costs and complexity. The versatility of using multi-substituted starting materials opens up an expansive horizon for the development of new target compounds, indicating that this synthesis strategy can cater to a wide array of chemical constituents.
The Broader Implications
Beyond its immediate applications in drug synthesis, the implications of this research could reshape how chemists approach organic synthesis as a whole. By establishing a strategy based on boron chemistry, this study paves the way for future explorations into organoboron compounds—previously seen as elusive within the chemistry community. The technique promises advancements in functionalizing complex aromatic systems, further enriching the toolbox available for researchers aiming to develop next-generation therapeutics.
The remarkable work emanating from Tokyo Tech embodies an essential stride in organic synthesis. By spotlighting the untapped potential of quinoline derivatives, this research not only enhances existing methodologies but also serves as a catalyst for future innovations in drug discovery. As chemists continue to explore these pathways, the possibilities for new treatments and therapies seem limitless, inviting a brighter future for medical science.