Recent findings from a research team led by Professor Jaeheung Cho at UNIST shed light on the intricate interplay between cobalt(III)-based metal complexes and nitrile substrates. Highlighted in the Journal of the American Chemical Society, this research delves into the reaction mechanisms employed by cobalt(III) within biomimetic frameworks. The significance of metal spin states emerges as a central theme, showcasing how these properties may significantly dictate the rates and results of chemical reactions.
The Role of Metal Spin States
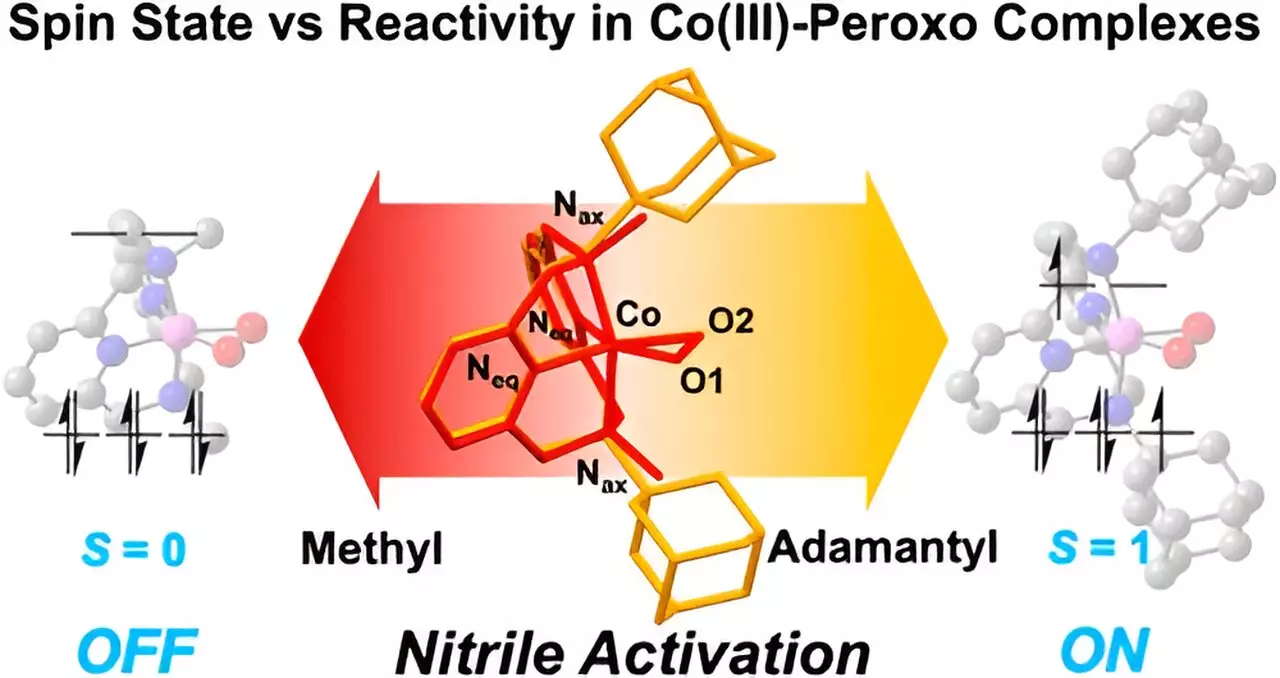
A critical aspect of this study is the emphasis on metal spin states, which are fundamental to understanding catalytic activity. Minor alterations in the properties of cobalt complexes reveal noticeable effects on reaction kinetics. This points to the necessity for precision in adjusting metal characteristics to manipulate the reactivity and efficiency of chemical processes. The ability to control these spin states is pivotal for developing novel reaction pathways that may lead to groundbreaking applications, particularly in pharmaceutical development.
To investigate the interaction between cobalt complexes and nitriles, the research team utilized a sophisticated framework known as the “Macrocyclic Pyridinophane System.” This highly adaptable structure allowed the scientists to innovate and modify the cobalt compounds effectively. A key outcome of using larger adamantyl groups was an observable escalation in nitrile activation reactions, indicating that size and configuration profoundly influence the chemistry at play. Conversely, the introduction of smaller methyl groups revealed a distinct lack of reactivity, further underscoring the importance of molecular structure in determining reaction efficiency.
Nitriles have garnered attention in various domains, particularly in pharmaceuticals and agrochemicals, owing to their versatile reactivity. However, the challenges posed by their reactivity profiles often limit their applications. The study’s findings that cobalt(III)-peroxo species can engage with nitriles at ambient temperatures to yield significant compounds illuminate a potential pathway toward new anticancer therapies. This discovery not only paves the way for innovative drug development but also hints at broader applications in medicinal chemistry.
The groundbreaking work led by Professor Cho and his team offers significant insights into the interplay between metal complexes and organic substrates, presenting valuable implications for future research directions. With cobalt(III) complexes demonstrating the ability to influence nitrile reactivity through tailored modifications, researchers can anticipate exciting advancements in chemical synthesis and drug discovery. Not only do these discoveries enhance our understanding of coordination chemistry, but they also contribute to the ongoing search for effective and innovative therapeutic agents in the fight against challenging diseases such as cancer. As the realm of metal-organic chemistry continues to evolve, this research stands as a compelling example of how fundamental science can pave the way to tangible real-world solutions.