Recent advances in material science have opened up new horizons, particularly in the realm of high entropy oxides (HEOs), which are emerging as pivotal components in modern electronic devices. A groundbreaking study published in the Journal of the American Chemical Society has illuminated the profound effects that various synthesis techniques can exert on the structural and functional attributes of these materials. High entropy oxides combine multiple transition metal oxides into a homogeneous structure, exhibiting remarkable electrochemical properties. Alannah Hallas, a material scientist at the University of British Columbia, emphasizes the versatility of these materials, stating that their “enormous chemical flexibility” provides a unique opportunity for innovation in their applications.
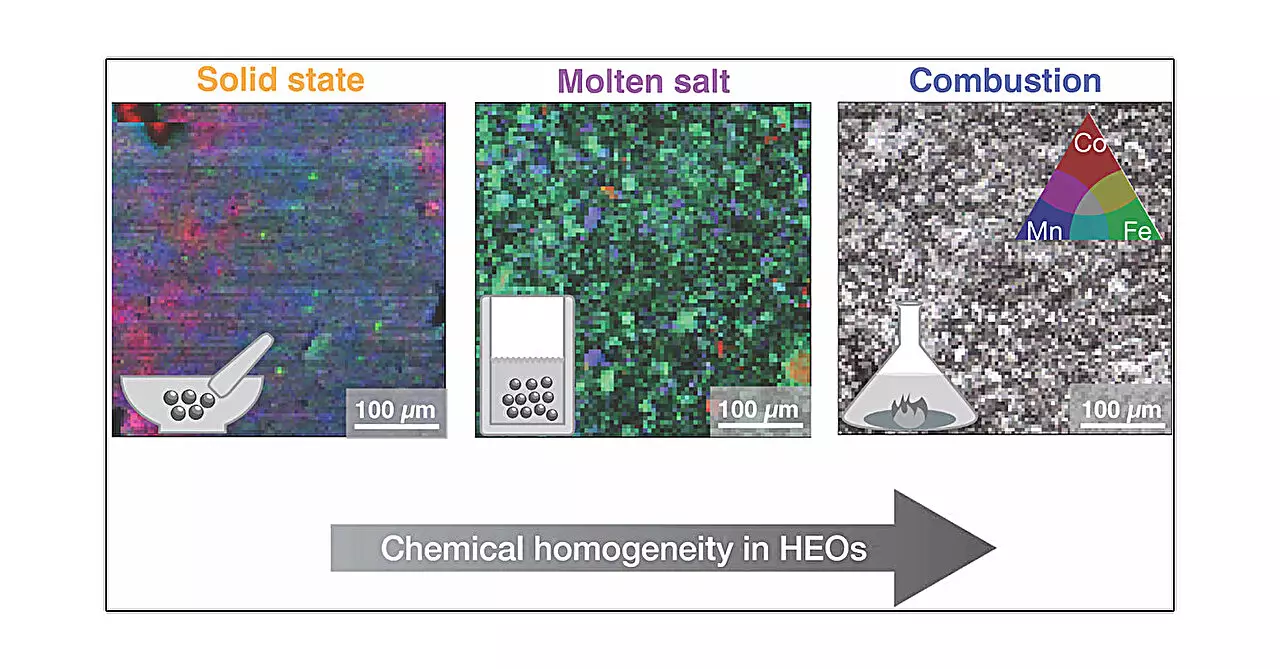
The study draws its insights from a meticulous examination of five distinct synthesis methods: solid-state synthesis, high-pressure synthesis, hydrothermal synthesis, molten salt synthesis, and combustion synthesis. Each of these techniques involves unique processes of heating, cooling, and chemical conditions that significantly influence the resulting materials. This comprehensive approach enables researchers to observe how variations in the synthetic environment can lead to substantial differences in microstructural and local properties, even if the overall structure remains stable.
For instance, the solid-state method can be likened to baking a cake—mixing ingredients (metal oxides) and then applying heat. In comparison, the high-pressure method introduces external forces that alter the formation dynamics, potentially yielding different outcomes in terms of microstructure. Hydrothermal synthesis simulates geological processes, allowing growth conditions akin to those in nature, whereas molten salt synthesis creates a liquid milieu for crystallization. Lastly, the rapid reactions of combustion synthesis present another avenue for creating these complex materials. Such diverse methodologies highlight the intricate relationship between synthesis conditions and the resultant material properties.
A striking revelation from the study is the significance of the synthesis method used. Although the research found that the average structural characteristics of the HEOs remained consistent across samples, the local structural variations and microstructural differences were pronounced, particularly with the combustion synthesis method yielding the most homogeneous samples. Lead author Mario Ulises González-Rivas explains how each synthesis technique’s driving mechanisms govern the resultant morphology and arrangement of atoms within the material. This aspect is crucial, as minor changes in microstructure can lead to considerable shifts in functionality, especially in energy-related applications.
González-Rivas contends that understanding these variations provides a “new optimization axis,” suggesting that future applications of high entropy oxides can be improved by selecting appropriate synthesis methods. The implications of this research are vast, as it not only enhances our scientific knowledge but also has practical ramifications in fields such as energy production, storage, and electronic device manufacturing.
This research is a product of collaboration between Hallas’ team at UBC’s Blusson Quantum Matter Institute and two other distinguished institutions. Such partnerships showcase the importance of interdisciplinary approaches in advancing material science. The combination of expertise from different fields can lead to innovative breakthroughs and optimize the development of materials with tailored properties for specific applications.
As contemporary challenges in energy management and electronics continue to evolve, exploring the synthesis of high entropy oxides remains a promising avenue. The prospect of fine-tuning these materials through various synthesis methods could lead to the creation of more efficient energy systems and components with superior performance characteristics. The study exemplifies how foundational research can translate into real-world applications, paving the way for engineers and scientists to harness the full potential of high entropy oxides.
The study of high entropy oxides through varying synthesis methods underscores the intricacies of material development and its implications for technological advancement. By elucidating the relationship between synthesis techniques and material properties, researchers set the stage for significant innovations in electronic and energy systems. Moving forward, it will be vital to continue exploring these materials to unlock new applications and enhance their utility in addressing pressing global challenges.